Hyoscyamine
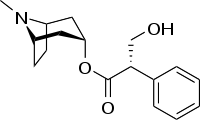
Hyoscyamine (also known as daturine or duboisine) is a naturally occurring tropane alkaloid and plant toxin. It is a secondary metabolite found in certain plants of the family Solanaceae, including henbane, mandrake, angel's trumpets, jimsonweed, tomato, the sorcerers' tree, and deadly nightshade. It is the levorotary isomer of atropine (third of the three major nightshade alkaloids) and thus sometimes known as levo-atropine.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anaspaz, Levbid, Levsin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684010 |
| Pregnancy category |
|
| Routes of administration | Oral, Injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50% protein binding |
| Metabolism | Hepatic |
| Elimination half-life | 3–5 hrs. |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.667 |
| Chemical and physical data | |
| Formula | C17H23NO3 |
| Molar mass | 289.375 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Brand names for hyoscyamine include Symax, HyoMax, Anaspaz, Egazil, Buwecon, Cystospaz, Levsin, Levbid, Levsinex, Donnamar, NuLev, Spacol T/S, and Neoquess.[2]
Uses
Hyoscyamine is used to provide symptomatic relief of spasms caused by various lower abdominal and bladder disorders including peptic ulcers, irritable bowel syndrome, diverticulitis, pancreatitis, colic, and interstitial cystitis.[3][4][5] It has also been used to relieve some heart problems, control some of the symptoms of Parkinson's disease, as well as for control of abnormal respiratory symptoms and "hyper-mucus secretions" in patients with lung disease.[6]
It is also useful in pain control for neuropathic pain, chronic pain and palliative care – "comfort care" – for those with intractable pain from treatment resistant, untreatable, and incurable diseases. When combined with opioids it increases the level of analgesia (pain relief) obtained.[7] Several mechanisms are thought to contribute to this effect. The closely related drugs atropine and hyoscine and other members of the anticholinergic drug group like cyclobenzaprine, trihexyphenidyl, and orphenadrine are also used for this purpose.[8] When hyoscyamine is used along with opioids or other anti-peristaltic agents, measures to prevent constipation are especially important given the risk of paralytic ileus.[9]
Adverse effects
Side effects include dry mouth and throat, increased appetite leading to weight gain, eye pain, blurred vision, restlessness, dizziness, arrhythmia, flushing, and faintness.[4] An overdose will cause headache, nausea, vomiting, and central nervous system symptoms including disorientation, hallucinations, euphoria, sexual arousal, short-term memory loss, and possible coma in extreme cases. The euphoric and sexual effects are stronger than those of atropine but weaker than those of hyoscine, as well as dicycloverine, orphenadrine, cyclobenzaprine, trihexyphenidyl, and ethanolamine antihistamines like phenyltoloxamine.[10][11]
Pharmacology
Hyoscyamine is an antimuscarinic; i.e., an antagonist of muscarinic acetylcholine receptors. It blocks the action of acetylcholine at parasympathetic sites in sweat glands, salivary glands, stomach secretions, heart muscle, sinoatrial node, smooth muscle in the gastrointestinal tract, and the central nervous system. It increases cardiac output and heart rate, lowers blood pressure and dries secretions.[12] It may antagonize serotonin.[13] At comparable doses, hyoscyamine has 98 per cent of the anticholinergic power of atropine. The other major belladonna-derived drug hyoscine (known in the United States as Scopolamine) has 92 per cent of the antimuscarinic potency of atropine.[13]
Biosynthesis in plants
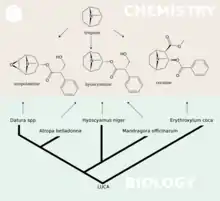
Hyoscyamine can be extracted from plants of the family Solanaceae, notably Datura stramonium. As hyoscyamine is a direct precursor in the plant biosynthesis of hyoscine, it is produced via the same metabolic pathway.[14]
The biosynthesis of hyoscine begins with the decarboxylation of L-ornithine to putrescine by ornithine decarboxylase (EC 4.1.1.17). Putrescine is methylated to N-methylputrescine by putrescine N-methyltransferase (EC 2.1.1.53).[14]
A putrescine oxidase (EC 1.4.3.10) that specifically recognizes methylated putrescine catalyzes the deamination of this compound to 4-methylaminobutanal which then undergoes a spontaneous ring formation to N-methylpyrrolium cation. In the next step, the pyrrolium cation condenses with acetoacetic acid yielding hygrine. No enzymatic activity could be demonstrated that catalyzes this reaction. Hygrine further rearranges to tropinone.[14]
Subsequently, tropinone reductase I (EC 1.1.1.206) converts tropinone to tropine which condenses with phenylalanine-derived phenyllactate to littorine. A cytochrome P450 classified as Cyp80F1[15] oxidizes and rearranges littorine to hyoscyamine aldehyde.

Bush medicine basis
A bush medicine developed by Aboriginal peoples of the eastern states of Australia from the soft corkwood tree, or Duboisia myoporoides, was used by the Allies in World War II to stop soldiers getting seasick when they sailed across the English Channel during the Invasion of Normandy. Later, it was found that the same substance could be used in the production of scopolamine and hyoscyamine, which are used in eye surgery, and a multi-million dollar industry was built in Queensland based on this substance.[16]
References
- Ushimaru R, Ruszczycky MW, Liu HW (January 2019). "Changes in Regioselectivity of H Atom Abstraction during the Hydroxylation and Cyclization Reactions Catalyzed by Hyoscyamine 6β-Hydroxylase". Journal of the American Chemical Society. 141 (2): 1062–1066. doi:10.1021/jacs.8b11585. PMC 6488026. PMID 30545219.
- "Hyoscyamine - brand name list from Drugs.com". Drugs.com. Archived from the original on 2022-08-20. Retrieved 2022-08-20.
- National Clinical Guideline Centre (UK) (2012). Treatment to improve bladder storage. NBK132836 (8th ed.). United Kingdom: Royal College of Physicians. p. 83 – via National Library of Medicine.
- "Hyoscyamine Uses, Side Effects & Warnings". Drugs.com. Archived from the original on 2022-08-20. Retrieved 2022-08-20.
- "Bladder Control Medicines | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Archived from the original on 2022-08-20. Retrieved 2022-08-20.
- "Hyoscyamine: MedlinePlus Drug Information". medlineplus.gov. Archived from the original on 2022-08-20. Retrieved 2022-08-20.
- Harden RN (March 2005). "Chronic neuropathic pain. Mechanisms, diagnosis, and treatment". The Neurologist. 11 (2): 111–122. doi:10.1097/01.nrl.0000155180.60057.8e. PMID 15733333. S2CID 12602416.
- Ali-Melkkilä T, Kanto J, Iisalo E (October 1993). "Pharmacokinetics and related pharmacodynamics of anticholinergic drugs". Acta Anaesthesiologica Scandinavica. 37 (7): 633–642. doi:10.1111/j.1399-6576.1993.tb03780.x. PMID 8249551. S2CID 22808654.
- Kamimura A, Howard S, Weaver S, Panahi S, Ashby J (December 2020). "The Use of Complementary and Alternative Medicine Strategies, Opioids, and Nonsteroidal Anti-Inflammatory Drugs (NSAIDS) Among Patients Attending a Free Clinic". Journal of Patient Experience. 7 (6): 1701–1707. doi:10.1177/2374373520937514. PMC 7786764. PMID 33457633.
- Kang M, Galuska MA, Ghassemzadeh S (2022). "Benzodiazepine Toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 29489152. Archived from the original on 2022-08-20.
- "Hyoscyamine Sulfate Sublingual Tablets, 0.125 mgRx Only". www.dailymed.nlm.nih.gov. Archived from the original on 2022-08-20. Retrieved 2022-08-20.
- Edwards Pharmaceuticals, Inc.; Belcher Pharmaceuticals, Inc. (May 2010). "DailyMed". U.S. National Library of Medicine. Retrieved January 13, 2013.
- Kapoor AK, Raju SM (2013). Illustrated Medical Pharmacology. JP Medical Ltd. p. 131. ISBN 9789350906552. Retrieved January 11, 2014.
- Ziegler J, Facchini PJ (2008). "Alkaloid biosynthesis: metabolism and trafficking". Annual Review of Plant Biology. 59 (1): 735–769. doi:10.1146/annurev.arplant.59.032607.092730. PMID 18251710.
- Li R, Reed DW, Liu E, Nowak J, Pelcher LE, Page JE, Covello PS (May 2006). "Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement". Chemistry & Biology. 13 (5): 513–520. doi:10.1016/j.chembiol.2006.03.005. PMID 16720272.
- "Visitors to Art of Healing exhibition told how Australian Indigenous bush medicine was given to every allied soldier landing at Normandy on D-Day". King's College London. 7 June 2019. Retrieved 2 June 2020.