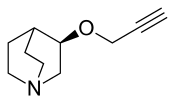
Talsaclidine
Talsaclidine (WAL-2014) is a non-selective muscarinic acetylcholine receptor agonist which acts as a full agonist at the M1 subtype, and as a partial agonist at the M2 and M3 subtypes.[1][2][3] It was under development for the treatment of Alzheimer's disease but showed only modest or poor efficacy in rhesus monkeys and humans, respectively,[3][4] perhaps due to an array of dose-limiting side effects including increased heart rate and blood pressure, increased salivation, urinary frequency and burning upon urination, increased lacrimation and nasal secretion, abnormal accommodation, heartburn, upset stomach as well as cramps, nausea, vomiting and diarrhea, excessive sweating and palpitations.[5]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Pharmacokinetic data | |
| Bioavailability | 70% |
| Protein binding | 7% |
| Excretion | Renal (86%) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.161.949 |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.236 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
See also
References
- Ensinger HA, Doods HN, Immel-Sehr AR, et al. (1993). "WAL 2014--a muscarinic agonist with preferential neuron-stimulating properties". Life Sciences. 52 (5–6): 473–80. doi:10.1016/0024-3205(93)90304-L. PMID 8441328.
- Walland A, Burkard S, Hammer R, Tröger W (1997). "In vivo consequences of M1-receptor activation by talsaclidine". Life Sciences. 60 (13–14): 977–84. doi:10.1016/S0024-3205(97)00037-4. PMID 9121364.
- Wienrich M, Meier D, Ensinger HA, et al. (April 2001). "Pharmacodynamic profile of the M1 agonist talsaclidine in animals and man". Life Sciences. 68 (22–23): 2593–600. doi:10.1016/S0024-3205(01)01057-8. PMID 11392631.
- Terry AV, Buccafusco JJ, Borsini F, Leusch A (July 2002). "Memory-related task performance by aged rhesus monkeys administered the muscarinic M(1)-preferring agonist, talsaclidine". Psychopharmacology. 162 (3): 292–300. doi:10.1007/s00213-002-1105-3. PMID 12122487. S2CID 23323985.
- Adamus WS, Leonard JP, Tröger W (1995). "Phase I clinical trials with WAL 2014, a new muscarinic agonist for the treatment of Alzheimer's disease". Life Sciences. 56 (11–12): 883–90. doi:10.1016/0024-3205(95)00024-Z. PMID 10188789.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.