Imidafenacin
Imidafenacin (INN) is a urinary antispasmodic of the anticholinergic class. Pharmacol:[1]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.216.585 |
| Chemical and physical data | |
| Formula | C20H21N3O |
| Molar mass | 319.40 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
- The sidechain is a versatile precursor also used in the synthesis of: Bezitramide, Piritramide, Diphenoxylate, & others.
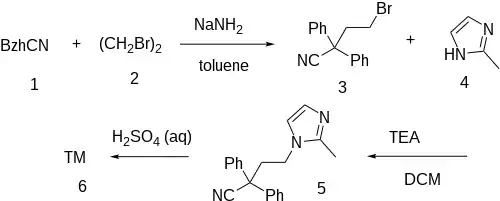
The alkylation of Diphenylacetonitrile [86-29-3] (1)[7] with 1,2-dibromoethane (2) gives 4-Bromo-2,2-Diphenylbutyronitrile [39186-58-8] (3). Alkylation with 2-Methylimidazole (4) gives 4-(2-Methyl-1H-imidazol-1-yl)-2,2-diphenylbutanenitrile [214777-43-2] (5). Partial hydrolysis of the nitrile to the amide completes the synthesis of Imidafenacin (6).
References
- Kobayashi F, Yageta Y, Segawa M, Matsuzawa S (2007). "Effects of imidafenacin (KRP-197/ONO-8025), a new anti-cholinergic agent, on muscarinic acetylcholine receptors. High affinities for M3 and M1 receptor subtypes and selectivity for urinary bladder over salivary gland". Arzneimittelforschung. 57 (2): 92–100. doi:10.1055/s-0031-1296589. PMID 17396619.
- Miyachi, Hiroyuki; Kiyota, Hiromi; Segawa, Mitsuru (1999). "Design, synthesis and antimuscarinic activity of some imidazolium derivatives". Bioorganic & Medicinal Chemistry Letters. 9 (20): 3003–3008. doi:10.1016/S0960-894X(99)00517-X.
- Yuuji Ishiguro, Yasuhiro Aizawa, Masahiro Aono, EP 1845091 (2007 to Kyorin Pharmaceutical Co., Ltd.).
- Hiroyuki Miyachi, Kei Okazaki, Hiromi Kiyota, Mitsuru Segawa, U.S. Patent 5,932,607 (1999 to Kyorin Pharmaceutical Co., Ltd.).
- Cai Hongfei, et al. CN 103772286 (2014 to Jiangxi Qingfeng Pharmaceutical Co Ltd).
- Jinchun Tang & Sunan Wang, CN1 02030682A (2010 to Changzhou Kangpu Pharmaceutical Co Ltd).
- Reid, Wm. B., Hunter, J. H. (October 1948). "Preparation of Diphenylacetonitrile". Journal of the American Chemical Society. 70 (10): 3515–3515. doi:10.1021/ja01190a509.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.