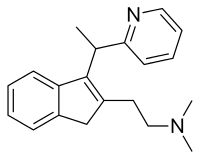
Dimetindene
Dimetindene , also sold under the brand name Fenistil, is an antihistamine/anticholinergic. It is a first generation[1] selective H1 antagonist.[2] Dimetindene is an atypical first generation H1 antagonist as it only minimally[3] passes across the blood-brain barrier.
 | |
| Clinical data | |
|---|---|
| Trade names | Fenistil |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, topical |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.024.622 |
| Chemical and physical data | |
| Formula | C20H24N2 |
| Molar mass | 292.426 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
SMILES
| |
InChI
| |
| (verify) | |
Dimetindene is also an M2 receptor antagonist.[4]
It was patented in 1958 and came into medical use in 1960.[5]
Medical use
Dimetindene is used orally and topically as an antipruritic.[6] It is used topically to treat skin irritations, such as insect bites. Dimetindene is also administered orally to treat allergies, such as hay fever.
It is commonly formulated as its maleic acid salt, dimethindene maleate.
Names
It is sold under the brand name Fenistil among others.
Synthesis
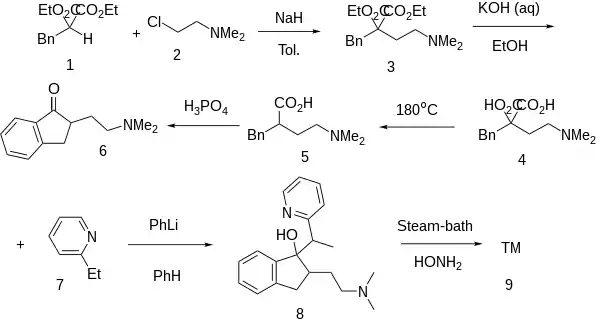
Note that Dolispan Fb: [1613-24-7] shares various of the first steps of the synthesis, indicating that 5 might already be pharmaceutically active in its own right.
The base catalyzed alkylation between Diethyl Benzylmalonate [607-81-8] (1) and 2-Chloroethyldimethylamine [107-99-3] (2) gives Diethyl 2-benzyl-2-(2-(dimethylamino)ethyl)malonate [1805-03-4] (3). Saponification of both esters gives 2-Benzyl-2-(2-(Dimethylamino)Ethyl)Malonic Acid [92501-97-8] (4). Decarboxylation of one of the acids leads to 2-Benzyl-4-(Dimethylamino)Butanoic Acid [1613-23-6] (5). A Haworth type intramolecular Friedel-Crafts acylation leads to 2-[2-(Dimethylamino)ethyl]-1-indanone [3409-21-0] (6). Base catalyzed alkylation of the carbonyl group with 2-Ethylpyridine [100-71-0] (7) gives CID:12963073 (8). Lastly, dehydration of the alcohol completes the synthesis of Dimethindene (9).
References
- Fromer LM, Ortiz GR, Dowdee AM (September 2008). "Assessment of Patient Attitudes About Mometasone Furoate Nasal Spray: The Ease-of-Use Patient Survey". The World Allergy Organization Journal. 1 (9): 156–9. doi:10.1186/1939-4551-1-9-145. PMC 3651039. PMID 23282579.
- "Dimetindene". Drugs.com.
- Noguchi; Inukai; Kuno; Tanaka (June 1992). "The suppression of olfactory bulbectomy-induced muricide by antidepressants and antihistamines via histamine H1 receptor blocking". Physiol. Behav. 51 (6): 1123–1127. doi:10.1016/0031-9384(92)90297-f. PMID 1353628. S2CID 29562845.
- Matsumoto Y, Miyazato M, Furuta A, Torimoto K, Hirao Y, Chancellor MB, Yoshimura N (April 2010). "Differential roles of M2 and M3 muscarinic receptor subtypes in modulation of bladder afferent activity in rats". Urology. 75 (4): 862–7. doi:10.1016/j.urology.2009.12.013. PMC 2871158. PMID 20156651.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 547. ISBN 9783527607495.
- Wexler L (June 1962). "A clinical evaluation of dimethindene". Current Therapeutic Research, Clinical and Experimental. 4: 306–9. PMID 14006402.
- Huebner, C. F., Donoghue, E., Wenk, P., Sury, E., Nelson, J. A. (April 1960). "A NEW CLASS OF HIGHLY ACTIVE ANTIHISTAMINICS". Journal of the American Chemical Society. 82 (8): 2077–2078. doi:10.1021/ja01493a060. ISSN 1520-5126 0002-7863, 1520-5126.
{{cite journal}}: Check|issn=value (help) - Charles Ferdinand Huebner, U.S. Patent 2,947,756 (1960 to Ciba).
- Huebner Charles Ferdinand, U.S. Patent 2,970,149 (1961 to CIBA PHARM PROD Inc).
- Huebner Charles Ferdinand, U.S. Patent 3,060,186 (1962 to Ciba Geigy Corp).