Alvameline
Alvameline (Lu 25-109) is a M1 receptor agonist and M2/M3 receptor antagonist[1] that was under investigation for the treatment of Alzheimer's disease, but produced poor results in clinical trials[2] and was subsequently discontinued.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H15N5 |
| Molar mass | 193.254 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Synthesis
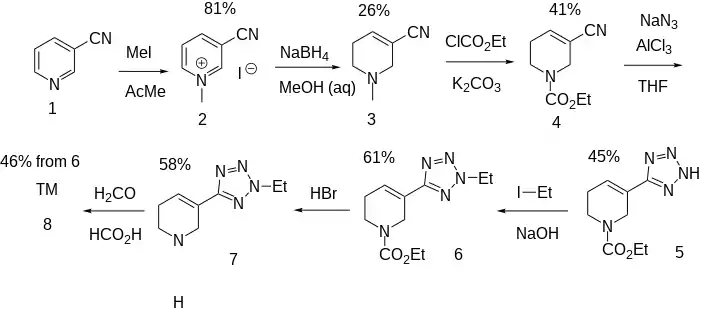
Alkylation of nicotinonitrile (accessible from nicotinamide)[5] (1) with methyl iodide affords the N-methylpyridinium salt (2). The reduction of this intermediate with sodium borohydride gives 3-cyano-N-methyl-1,2,5,6-tetrahydropyridine [5657-66-9] (3). Reaction with ethyl chloroformate results in N-demethylation and consequent formation of the corresponding carbamate [120241-16-9] (4). The nitrile group is then transformed to a tetrazole by reaction with sodium azide in the presence of aluminum chloride giving CID:9991151 (5). The surrogate acid is then alkylated with ethyl iodide to afford CID:10106197 (6). Treatment with acid then removes the carbamate on the ring nitrogen giving Lu-25-077 [221549-70-8] (7). The methyl group on the piperidine ring restored using formaldehyde and formic acid under standard Eschweiler–Clarke conditions, yielding alvameline (8).
See also
References
- Sánchez C, Arnt J, Didriksen M, Dragsted N, Moltzen Lenz S, Matz J (June 1998). "In vivo muscarinic cholinergic mediated effects of Lu 25-109, a M1 agonist and M2/M3 antagonist in vitro". Psychopharmacology. 137 (3): 233–40. doi:10.1007/s002130050615. PMID 9683000. S2CID 20740372. Archived from the original on 2000-10-02. Retrieved 2009-12-03.
- Sramek JJ, Forrest M, Mengel H, Jhee SS, Hourani J, Cutler NR (1998). "A bridging study of LU 25-109 in patients with probable Alzheimer's disease". Life Sciences. 62 (3): 195–202. doi:10.1016/S0024-3205(97)01087-4. PMID 9488097.
- Moltzen, E. K.; Pedersen, H.; Boegesoe, K. P.; Meier, E.; Frederiksen, K.; Sanchez, C.; Lemboel, H. L. (1994). "Bioisosteres of Arecoline: 1,2,3,6-Tetrahydro-5-pyridyl-Substituted and 3-Piperidyl-Substituted Derivatives of Tetrazoles and 1,2,3-Triazoles. Synthesis and Muscarinic Activity". Journal of Medicinal Chemistry. 37 (24): 4085–4099. doi:10.1021/jm00050a006. PMID 7990109.
- EP0296721 idem Klaus P. Bogeso, Klaus G. Jensen, Ejner K. Moltzen, Henrik Pedersen, U.S. Patent 4,866,077 (1988 to H Lundbeck AS).
- "Nicotinonitrile". Organic Syntheses. 33: 52. 1953. doi:10.15227/orgsyn.033.0052.; Collective Volume, vol. 4, p. 706