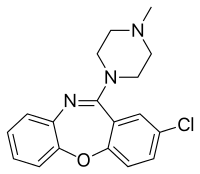
Loxapine
Loxapine, sold under the brand names Loxitane and Adasuve (inhalation only) among others, is an antipsychotic medication used primarily in the treatment of schizophrenia. The medicine is a member of the dibenzoxazepine class and structurally very similar to clozapine. Several researchers have argued that loxapine, initially classified as a typical antipsychotic, behaves as an atypical antipsychotic.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Loxitane, Adasuve |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682311 |
| License data | |
| Routes of administration | By mouth, inhalation, intramuscular |
| Drug class | Antipsychotic |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96.8%[1] |
| Metabolism | Extensive Liver; active metabolites include amoxapine and 8-hydroxyloxapine. Inhibits P-gp and is a substrate of CYP1A2, CYP3A4 and CYP2D6[1] |
| Elimination half-life | 4 hours (oral); 7.61 hours (inhalation)[1] |
| Excretion | Majority are excreted within 24 hours, main route through urine (conjugated metabolites), small amounts through the feces (unconjugated metabolites) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.215 |
| Chemical and physical data | |
| Formula | C18H18ClN3O |
| Molar mass | 327.81 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 109 to 110 °C (228 to 230 °F) |
SMILES
| |
InChI
| |
| | |

Loxapine may be metabolized by N-demethylation to amoxapine, a tricyclic antidepressant.[3]
Medical uses
The US Food and Drug Administration (FDA) has approved loxapine inhalation powder for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults.[4]
A brief review of loxapine found no conclusive evidence that it was particularly effective in patients with schizophrenia.[5] A subsequent systematic review considered that the limited evidence did not indicate a clear difference in its effects from other antipsychotics.[6]
Side effects
Loxapine can cause side effects that are generally similar to that of other antipsychotic medications. These include, e.g., gastrointestinal problems (like constipation and abdominal pain), cardiovascular problems (like tachycardia), moderate likelihood of drowsiness (relative to other antipsychotics),[8] and movement problems (i.e. extrapyramidal symptoms [EPS]).[9] At lower dosages its propensity for causing EPS appears to be similar to that of atypical antipsychotics.[10] Although it is structurally similar to clozapine, it has much lower risk of agranulocytosis (which, even with clozapine, is 0.8%); however, mild and temporary fluctuations in blood leukocyte levels can occur.[11][12] Abuse of loxapine has been reported.[13]
The inhaled formulation of loxapine carries a low risk for a type of airway adverse reaction called bronchospasm that is not thought to occur when loxapine is taken by mouth.[4]
Pharmacology
Mechanism of action
Some authors say loxapine is a "mid-potency" typical antipsychotic.[12] However, unlike most other typical antipsychotics, it has significant potency at the 5HT2A receptor (6.6 nM), which is similar to atypical antipsychotics like clozapine (5.35 nM). The higher likelihood of EPS with loxapine, compared to clozapine, may be due to its higher affinity for the D2 receptor compared to clozapine, which has one of the lowest binding affinities at the D2 receptor of any antipsychotic.[12]
| Site | LOX | AMX |
|---|---|---|
| 5-HT1A | 2,460 | ND |
| 5-HT1B | 388 | ND |
| 5-HT1D | 3,470 | ND |
| 5-HT1E | 1,400 | ND |
| 5-HT2A | 6.6 | 0.5 |
| 5-HT2C | 13 | 2 (rat) |
| 5-HT3 | 190 | ND |
| 5-HT5A | 780 | ND |
| 5-HT6 | 31 | 50 |
| 5-HT7 | 88 | 40 (rat) |
| α1A | 31 | ND |
| α1B | 53 | ND |
| α2A | 151 | ND |
| α2B | 108 | ND |
| α2C | 80 | ND |
| β1 | >10,000 | ND |
| β2 | >10,000 | ND |
| M1 | 120 | ND |
| M2 | 445 | ND |
| M3 | 211 | ND |
| M4 | 1,270 | ND |
| M5 | 166 | ND |
| D1 | 54 | ND |
| D2 | 11 | 21 |
| D3 | 19 | 21 |
| D4 | 8.4 | 21 |
| D5 | 75 | ND |
| H1 | 2.2–4.9 | 7.9–25 |
| H2 | 208 | ND |
| H3 | 55,000 | >100,000 |
| H4 | 5,050–8,710 | 6,310 |
| SERT | >10,000 | 58 |
| NET | 5,700 | 16 |
| DAT | >10,000 | 58 |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||
Pharmacokinetics
Loxapine is metabolized to amoxapine, as well as its 8-hydroxy metabolite (8-hydroxyloxapine).[1] Amoxapine is further metabolized to its 8-hydroxy metabolite (8-hydroxyamoxapine), which is also found in the blood of people taking loxapine.[16] At steady-state after taking loxapine by mouth, the relative amounts of loxapine and its metabolites in the blood is as follows: 8-hydroxyloxapine > 8-hydroxyamoxapine > loxapine.[16]
The pharmacokinetics of loxapine change depending on how it is given. Intramuscular injections of loxapine lead to higher blood levels and area under the curve of loxapine than when it is taken by mouth.[16]
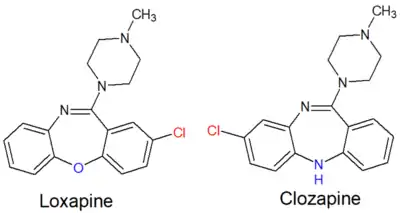
Chemistry
Loxapine is a dibenzoxazepine and is structurally very similar to clozapine, an atypical antipsychotic.
References
- Truven Health Analytics, Inc. DrugPoint System (Internet) [cited 2013 Sep 21]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- Glazer WM (1999). "Does loxapine have "atypical" properties? Clinical evidence". The Journal of Clinical Psychiatry. 60 (Suppl 10): 42–6. PMID 10340686.
- Cheung SW, Tang SW, Remington G (March 1991). "Simultaneous quantitation of loxapine, amoxapine and their 7- and 8-hydroxy metabolites in plasma by high-performance liquid chromatography". Journal of Chromatography. 564 (1): 213–21. doi:10.1016/0378-4347(91)80083-O. PMID 1860915.
- "ADASUVE Package Insert" (PDF). Galen US Inc.
- "Clozapine and loxapine for schizophrenia". Drug and Therapeutics Bulletin. 29 (11): 41–2. May 1991. doi:10.1136/dtb.29.11.41. PMID 1747161. S2CID 27613339.
- Chakrabarti A, Bagnall A, Chue P, et al. (2007). Chakrabarti A (ed.). "Loxapine for schizophrenia". Cochrane Database of Systematic Reviews (4): CD001943. doi:10.1002/14651858.CD001943.pub2. PMC 7017975. PMID 17943763.
- "LOXITANE Package Insert" (PDF). Watson Laboratories, Inc.
- Taylor D, Paton C, Kapur S, Taylor D, South London and Maudsley NHS Trust. The Maudsley prescribing guidelines in psychiatry [Internet]. Chichester, West Sussex: John Wiley & Sons; 2012 [cited 2013 Sep 21]. Available from: http://site.ebrary.com/lib/uqat/Doc?id=10531429
- Chakrabarti, Abhijit; Bagnall, Anne-Marie; Chue, Pierre; Fenton, Mark; Palanisamy, Vikram; Wong, Winson; Xia, Jun (17 October 2007). "Loxapine for schizophrenia". Cochrane Database of Systematic Reviews (4): CD001943. doi:10.1002/14651858.CD001943.pub2. PMC 7017975. PMID 17943763.
- Nordstrom K. Inhaled loxapine for rapid treatment of agitation in schizophrenia and bipolar disorder: an update. Neuropsychiatry [Internet]. 2012 Jun [cited 2013 Sep 21];2(3):253–60. Available from: Nordstrom, Kimberly (2012). "Inhaled loxapine for rapid treatment of agitation in schizophrenia and bipolar disorder: An update". Neuropsychiatry. 2 (3): 253–260. doi:10.2217/npy.12.23. S2CID 39718567.
- DePaulo, J. Raymond; Ayd, Frank J. (March 1982). "Loxapine: Fifteen years' clinical expenence". Psychosomatics. 23 (3): 261–271. doi:10.1016/S0033-3182(82)73416-4. PMID 7041162.
- Singh, AN; Barlas, C; Singh, S; Franks, P; Mishra, RK (January 1996). "A neurochemical basis for the antipsychotic activity of loxapine: interactions with dopamine D1, D2, D4 and serotonin 5-HT2 receptor subtypes". Journal of Psychiatry & Neuroscience. 21 (1): 29–35. PMC 1188731. PMID 8580115.
- Sperry L, Hudson B, Chan CH (March 1984). "Loxapine abuse". The New England Journal of Medicine. 310 (9): 598. doi:10.1056/NEJM198403013100920. PMID 6694719.
- Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Appl H, Holzammer T, Dove S, Haen E, Strasser A, Seifert R (2012). "Interactions of recombinant human histamine H1R, H2R, H3R, and H4R receptors with 34 antidepressants and antipsychotics". Naunyn Schmiedebergs Arch. Pharmacol. 385 (2): 145–70. doi:10.1007/s00210-011-0704-0. PMID 22033803. S2CID 14274150.
- Simpson, George M.; Cooper, Thomas B.; Lee, J. Hillary; Young, Michael A. (1978). "Clinical and plasma level characteristics of intramuscular and oral loxapine". Psychopharmacology. 56 (2): 225–232. doi:10.1007/BF00431855. PMID 417377. S2CID 21795809.