2CBFly-NBOMe
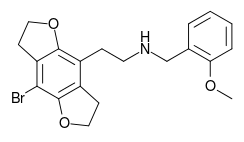
2CBFly-NBOMe (NBOMe-2C-B-FLY, Cimbi-31) is a compound indirectly derived from the phenethylamine hallucinogen 2C-B, and related to benzodifurans like 2C-B-FLY and N-benzylphenethylamines like 25I-NBOMe. It was discovered in 2002,[1] and further researched by Ralf Heim at the Free University of Berlin,[2] and subsequently investigated in more detail by a team at Purdue University led by David E. Nichols.[3] It acts as a potent partial agonist for the 5HT2A serotonin receptor subtype.[4][5][6]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(8-Bromo-2,3,6,7-tetrahydrobenzo[1,2-b:4,5-b′]difuran-4-yl)-N-[(2-methoxyphenyl)methyl]ethan-1-amine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| Abbreviations | 2CBFly-NBOMe |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C20H22BrNO3 |
| Molar mass | 404.298 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Analogues and derivatives
Analogues and derivatives of 2C-B:
25-N:
- 25B-N1POMe
- 25B-NAcPip
25-NM:
- 25B-NMe7BF
- 25B-NMe7BT
- 25B-NMe7Box
- 25B-NMe7DHBF
- 25B-NMe7Ind
- 25B-NMe7Indz
- 25B-NMePyr
- 2C-B-DRAGONFLY
- 2C-B-FLY
- 2CBFly-NBOMe (NBOMe-2CB-Fly)
- DOB-FLY
Other:
- 2C-B-AN
- 2C-B-BUTTERFLY
- 2C-B-FLY-NB2EtO5Cl
- 2CB-5-hemifly
- 2CB-Ind
- βk-2C-B (beta-keto 2C-B)
- N-Ethyl-2C-B
- TCB-2 (2C-BCB)
- BOB_(psychedelic)
- Β-Methyl-2C-B
- 2C-B-aminorex
- 2C-B-PP
- 2C-B-BZP
- DMBMPP
Legality
United Kingdom
This substance is a Class A drug in the United Kingdom as a result of the N-benzylphenethylamine catch-all clause in the Misuse of Drugs Act 1971.[7]
United States
2CBFly-NBOMe is a controlled substance in Vermont as of January 2016.[8]
References
- Elz S, Klass T, Heim R, Warnke U, Pertz HH (2002). "Development of highly potent partial agonists and chiral antagonists as tools for the study of 5-HT2A-receptor mediated function". Naunyn-Schmiedeberg's Archives of Pharmacology. 365 (1 Suppl): R21–R40. doi:10.1007/s00210-002-0604-4.
- Heim R (2004). Synthese und Pharmakologie potenter 5-HT2A-Rezeptoragonisten mit N-2-Methoxybenzyl-Partialstruktur. Entwicklung eines neuen Struktur-Wirkungskonzepts (PhD.). Free University of Berlin.
- Braden MR (2007). Towards a biophysical understanding of hallucinogen action (PhD.). Purdue University. ProQuest 304838368.
- Silva ME, Heim R, Strasser A, Elz S, Dove S (January 2011). "Theoretical studies on the interaction of partial agonists with the 5-HT2A receptor". Journal of Computer-Aided Molecular Design. 25 (1): 51–66. Bibcode:2011JCAMD..25...51S. CiteSeerX 10.1.1.688.2670. doi:10.1007/s10822-010-9400-2. PMID 21088982. S2CID 3103050.
- Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, et al. (April 2011). "Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers". European Journal of Nuclear Medicine and Molecular Imaging. 38 (4): 681–93. doi:10.1007/s00259-010-1686-8. PMID 21174090. S2CID 12467684.
- Hansen M (2011). Design and Synthesis of Selective Serotonin Receptor Agonists for Positron Emission Tomography Imaging of the Brain (PhD.). University of Copenhagen. Archived from the original on 2013-10-22. Retrieved 2012-11-02.
- "The Misuse of Drugs Act 1971 (Ketamine etc.) (Amendment) Order 2014". www.legislation.gov.uk.
- "Regulated Drugs Rule" (PDF). Vermont Department of Health. Retrieved 14 October 2015.