Vabicaserin
Vabicaserin (codenamed SCA-136) was a novel antipsychotic and anorectic under development by Wyeth.[1] As of 2010 it is no longer in clinical trials for the treatment of psychosis.[1][2] It was also under investigation as an antidepressant but this indication appears to have been dropped as well.[3]
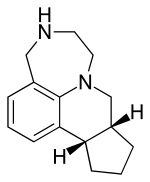 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H21ClN2 |
| Molar mass | 264.80 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Vabicaserin acts as a selective 5-HT2C receptor full agonist (Ki = 3 nM; EC50 = 8 nM; IA = 100% (relative to 5-HT)) and 5-HT2B receptor antagonist (IC50 = 29 nM).[4][5][6] It is also a very weak antagonist at the 5-HT2A receptor (IC50 = 1,650 nM), though this action is not clinically significant.[4] By activating 5-HT2C receptors, vabicaserin inhibits dopamine release in the mesolimbic pathway, likely underlying its efficacy in alleviating positive symptoms of schizophrenia, and increases acetylcholine and glutamate levels in the prefrontal cortex, suggesting benefits against cognitive symptoms as well.[6][7][8]
See also
References
- "Search of: vabicaserin - List Results - ClinicalTrials.gov".
- Lu, Chuang; Li, Albert P. (26 January 2010). Enzyme Inhibition in Drug Discovery ... - Google Books. ISBN 9780470538944.
- Prof John Kelly (2010). Principles of CNS Drug Development: From Test Tube to Patient. New York: Wiley. ISBN 978-0-470-51979-0.
- Rosenzweig-Lipson S, Dunlop J, Marquis KL (November 2007). "5-HT2C receptor agonists as an innovative approach for psychiatric disorders". Drug News & Perspectives. 20 (9): 565–71. doi:10.1358/dnp.2007.20.9.1162244. PMID 18176661.
- Tong Z, Chandrasekaran A, Demaio W, et al. (December 2009). "Species Differences in the Formation of Vabicaserin Carbamoyl Glucuronide". Drug Metabolism and Disposition. 38 (4): 581–590. doi:10.1124/dmd.109.028639. PMID 20032194. S2CID 793693.
- "ECNP-2007 CIS".
- Stahl's essential psychopharmacology: neuroscientific basis and practical applications. Cambridge, UK: Cambridge University Press. 2008. ISBN 978-0-521-85702-4.
- Albert, JS (2012). Wood, MW (ed.). Targets and Emerging Therapies for Schizophrenia. Hoboken, New Jersey: John Wiley & Sons, Inc. ISBN 9781118309384.