Ro 04-6790
Ro 04-6790 is a drug, developed by Hoffmann–La Roche, which has applications in scientific research. It acts as a potent and selective receptor antagonist for the 5-HT6 serotonin receptor subtype, with little or no affinity at other receptors. In common with other drugs of this class,[1] Ro 04-6790 has nootropic effects in animals,[2] and reduces the amnesia produced by memory-impairing drugs such as dizocilpine[3] and scopolamine.[4]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
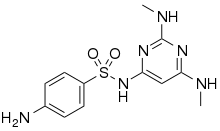
| Formula | C12H16N6O2S |
| Molar mass | 308.36 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
References
- Fone KC (November 2008). "An update on the role of the 5-hydroxytryptamine6 receptor in cognitive function". Neuropharmacology. 55 (6): 1015–22. doi:10.1016/j.neuropharm.2008.06.061. PMID 18655798. S2CID 35522597.
- King MV, Spicer CH, Sleight AJ, Marsden CA, Fone KC (January 2009). "Impact of regional 5-HT depletion on the cognitive enhancing effects of a typical 5-ht(6) receptor antagonist, Ro 04-6790, in the Novel Object Discrimination task". Psychopharmacology. 202 (1–3): 111–23. doi:10.1007/s00213-008-1334-1. PMID 18839151. S2CID 10826081.
- Pitsikas N, Zisopoulou S, Pappas I, Sakellaridis N (April 2008). "The selective 5-HT(6) receptor antagonist Ro 04-6790 attenuates psychotomimetic effects of the NMDA receptor antagonist MK-801". Behavioural Brain Research. 188 (2): 304–9. doi:10.1016/j.bbr.2007.11.010. PMID 18164078. S2CID 40183481.
- Woolley ML, Marsden CA, Sleight AJ, Fone KC (December 2003). "Reversal of a cholinergic-induced deficit in a rodent model of recognition memory by the selective 5-HT6 receptor antagonist, Ro 04-6790" (PDF). Psychopharmacology. 170 (4): 358–67. doi:10.1007/s00213-003-1552-5. PMID 13680084. S2CID 25193679.
| AChE inhibitor medications | |
|---|---|
| Other medications | |
| Experimental BACE inhibitors | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.