Pirenperone
Pirenperone (INN, USAN, BAN; developmental code names R-47456, R-50656) is a serotonin receptor antagonist described as an antipsychotic and tranquilizer which was never marketed.[1][2] It is a relatively selective antagonist of the serotonin 5-HT2 receptors and has been used in scientific research to study the serotonin system.[2][3] In the 1980s, the drug was found to block the effects of the lysergic acid diethylamide (LSD) in animals, and along with ketanserin, led to the elucidation of the 5-HT2A receptor as the biological mediator of the effects of serotonergic psychedelics.[4]
 | |
| Clinical data | |
|---|---|
| Other names | R-47456; R-50656 |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.071.081 |
| Chemical and physical data | |
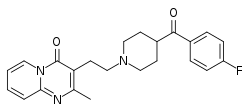
| Formula | C23H24FN3O2 |
| Molar mass | 393.462 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
The sidechain is formed from the cyclization of 2-aminopyridine with 2-Acetylbutyrolactone (2) in PPA gave 3-(2-hydroxyethyl)-2-methylpyrido[1,2-a]pyrimidin-4-one [41078-67-5] (3). Halogenation replaces the hydroxy with the chloride leaving group. Many syntheses do both steps in one-pot with phosphorus oxychloride.
The 4-(4-Fluorobenzoyl)Piperidine [56346-57-7] has 12 known named uses.
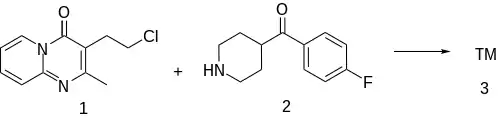
The alkylation between 3-(2-chloroethyl)-2-methylpyrido[1,2-a]pyrimidin-4-one [41078-70-0] (1) and 4-(4-fluorobenzoyl)piperidine hydrochloride [25519-78-2] (2) afforded pirenperone (3).
See also
- Altanserin
- Ketanserin
- Setoperone
- Risperidone
- Ocaperidone
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 994–. ISBN 978-1-4757-2085-3.
- I.K. Morton; Judith M. Hall (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 223–. ISBN 978-0-7514-0499-9.
- Francis C. Colpaert; Robert Balster (6 December 2012). Transduction Mechanisms of Drug Stimuli. Springer Science & Business Media. pp. 29–. ISBN 978-3-642-73223-2.
- Nichols DE (2016). "Psychedelics". Pharmacol. Rev. 68 (2): 264–355. doi:10.1124/pr.115.011478. PMC 4813425. PMID 26841800.
- EP0037265 idem Ludo E. J. Kennis, Josephus C. Mertens; U.S. Patent 4,342,870 (1982 to Janssen Pharmaceutica N.V.).