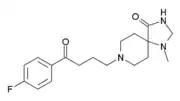
KML-010
KML-010 is a drug derived from spiperone. It functions as a highly selective 5-HT2A receptor antagonist, with negligible affinity for the 5-HT1A or 5-HT2C receptors, and over 400-fold lower affinity for the D2 receptor in comparison to spiperone.[1]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C18H24FN3O2 |
| Molar mass | 333.407 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
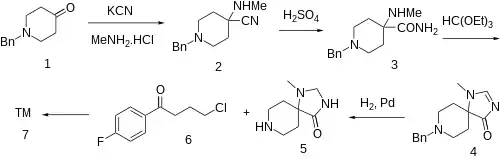
Synthesis
The Strecker condensation between N-Benzyl-4-piperidone [3612-20-2] (1), KCN and MeNH2.HCl produces amino nitrile, 1-Benzyl-4-(methylamino)piperidine-4-carbonitrile [953-79-7] (2). Partial hydrolysis of nitrile with H2SO4 furnishes amide, 1-benzyl-4-(methylamino)piperidine-4-carboxamide [1024-11-9] (3). Condensation of the amino amide with triethyl orthoformate gives rise to the spiro imidazolinone, 8-Benzyl-1-methyl-1,3,8-triazaspiro[4.5]decan-4-one, CID:10539225 (4). Catalytic hydrogenation of the imidazoline double bond of and simultaneous N-benzyl group hydrogenolysis gives rise to 1-methyl-1,3,8-triazaspiro[4,5]decan-4-one [701897-99-6][219563-51-6] (5). Alkylation with 4-chloro-4'-fluorobutyrophenone [3874-54-2] (6) completed the synthesis of KML-010 (7).
See also
References
- Glennon RA, Metwally K, Dukat M, Ismaiel AM, De los Angeles J, Herndon J, Teitler M, Khorana N (June 2002). "Ketanserin and spiperone as templates for novel serotonin 5-HT(2A) antagonists". Current Topics in Medicinal Chemistry. 2 (6): 539–58. doi:10.2174/1568026023393787. PMID 12052193.
- Metwally, Kamel A.; Dukat, Malgorzata; Egan, Christina T.; Smith, Carol; DuPre, Ann; Gauthier, Colleen B.; Herrick-Davis, Katharine; Teitler, Milt; Glennon, Richard A. (1998). "Spiperone: Influence of Spiro Ring Substituents on 5-HT2ASerotonin Receptor Binding". Journal of Medicinal Chemistry. 41 (25): 5084–5093. doi:10.1021/jm980452a.
- Janssen Paul Adriaan Jan, U.S. Patent 3,155,669 (1964 to Research Laboratorium C Janssen NV).