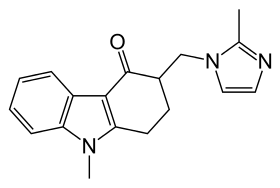
Ondansetron
Ondansetron, sold under the brand name Zofran among others, is a medication used to prevent nausea and vomiting caused by cancer chemotherapy, radiation therapy, or surgery.[9] It is also effective for treating gastroenteritis.[10][11] It can be given by mouth or by injection into a muscle or into a vein.[9]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zofran, Atossa,[1] others[2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601209 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, rectal, intravenous, intramuscular, thin film |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | ~60% |
| Protein binding | 70–76% |
| Metabolism | Liver (CYP3A4, CYP1A2, CYP2D6) |
| Elimination half-life | 5.7 hours |
| Excretion | Kidney |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.110.918 |
| Chemical and physical data | |
| Formula | C18H19N3O |
| Molar mass | 293.370 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Common side effects include diarrhea, constipation, headache, sleepiness, and itchiness.[9] Serious side effects include QT prolongation and severe allergic reaction.[9] It appears to be safe during pregnancy but has not been well studied in this group.[9] It is a serotonin 5-HT3 receptor antagonist.[9] It does not have any effect on dopamine receptors or muscarinic receptors.[12]
Ondansetron was patented in 1984 and approved for medical use in 1990.[13] It is on the World Health Organization's List of Essential Medicines.[14] It is available as a generic medication.[9] In 2020, it was the 83rd most commonly prescribed medication in the United States, with more than 8 million prescriptions.[15][16]
Medical uses
Although an effective antiemetic agent, the high cost of brand-name ondansetron initially limited its use to controlling postoperative nausea and vomiting and chemotherapy-induced nausea and vomiting.[17]
Cancer treatment
The 5-HT3 receptor antagonists are the primary medications used to treat and prevent chemotherapy-induced nausea and vomiting and radiotherapy-induced nausea and vomiting.
Postoperative
A number of medications including ondansetron appear to be effective in controlling postoperative nausea and vomiting. It is more effective than metoclopramide, and less sedating than cyclizine or droperidol.
Pregnancy
Ondansetron is used off-label to treat morning sickness and hyperemesis gravidarum of pregnancy. It is typically used after other antinausea drugs have failed.[18]
There appears to be a low risk of harm to the baby with use during pregnancy, though there may be an increase in heart problems among the babies.[19][20]
Ondansetron is in pregnancy category B in the US.[7][3] It is not known if ondansetron is excreted in breast milk.[7][3]
Cyclic vomiting syndrome
Ondansetron is one of several antiemetic drugs used during the vomiting phase of cyclic vomiting syndrome.[21]
Gastroenteritis
Trials in emergency department settings support the use of ondansetron to reduce vomiting associated with gastroenteritis and dehydration.[22] A retrospective review found it was used commonly for this purpose, being administered in over 58% of cases. Its use reduced hospital admissions, but was also associated with higher rates of return visits to the emergency department. Furthermore, people who had initially received ondansetron were more likely to be admitted on the return visit than people who had not received the drug. However, this effect may simply be due to the agent being used more frequently in people who present with more severe illness. Its use was not found to mask serious diagnoses.[23]
Irritable Bowel Syndrome (IBS)
In one study of patients with IBS-D variant ondansetron showed statistically significant effect on stool consistency and bloating. Nonetheless, it did not have any antinociceptive activity.[24]
Special populations
Children
Ondansetron has rarely been studied in people under 4 years of age. As such, little data is available to guide dosage recommendations.[7]
Elderly
It is not necessary to adjust the dosage for people under 75 years of age. The use of ondansetron has not been studied in people older than 75 years of age, and it is not known if dosage should be adjusted for this group.[7]
Poor liver function
The maximum recommended dose for people with severe liver function impairment is 8 mg/day. In these people, ondansetron is cleared from the body at half to one-third the rate as in healthy people. The concentration of ondansetron in body tissues as opposed to plasma is also higher than in healthy people.[7]
Adverse effects
Headache is the most common adverse effect.[7] A review of use for post-operative nausea and vomiting found that for every 36 people treated, one would experience headache, which could be severe.[25]
Constipation, diarrhea, and dizziness are other commonly reported side effects.[9] It is broken down by the hepatic cytochrome P450 system and it has little effect on the metabolism of other drugs broken down by this system. Anecdotally, ototoxicity has also been reported if injected too quickly.[9]
QT prolongation
Use of ondansetron has been associated with prolongation of the QT interval, which can lead to a potentially fatal heart rhythm known as torsades de pointes. Although this may happen in any person with any formulation, the risk is most salient with the injectable (intravenous) form of the drug and increases with dose. The risk is also higher in people taking other medicines that prolong the QT interval, as well as in people with congenital long QT syndrome, congestive heart failure, and/or bradyarrhythmias. As such, single doses of injectable ondansetron should not exceed 16 mg at one time. (Oral dosing recommendations remain intact, including the recommendation of a single 24-mg oral dose when indicated.) Electrolyte imbalances should be corrected before the use of injectable ondansetron. People are cautioned to seek immediate medical care if symptoms such as irregular heartbeat/palpitations, shortness of breath, dizziness, or fainting occur while taking ondansetron.[26]
Overdose
No specific treatment is available for ondansetron overdose; people are managed with supportive measures. An antidote to ondansetron is not known.[7]
Pharmacology
Pharmacodynamics
Ondansetron is a highly selective serotonin 5-HT3 receptor antagonist, with low affinity for dopamine receptors. The 5-HT3 receptors are present both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema in the medulla. Serotonin is released by the enterochromaffin cells of the small intestine in response to chemotherapeutic agents and may stimulate vagal afferents (via 5-HT3 receptors) to initiate the vomiting reflex. It is thought that ondansetron's antiemetic action is mediated mostly via antagonism of vagal afferents with a minor contribution from antagonism of central receptors.[27]
Pharmacokinetics
Ondansetron may have a degree of peripheral selectivity due to binding to P-glycoprotein and efflux out of the brain at the blood–brain barrier.[28][29][30]
History
.JPG.webp)
Ondansetron (marketed under the brand name Zofran) was developed in the mid-1980s by GlaxoSmithKline in London. It was granted US patent protection in September 1987,[31] received a use patent June 1988,[32] and was approved by the US FDA in January 1991. It was granted another divisional patent in November 1996.[33] Finally, owing to GlaxoSmithKline's research on pediatric use, ondansetron's patent protection was extended until December 2006.[34] By this final year of its patent (2006), Zofran had become the 20th highest-selling brand-name drug in the United States, with sales of US$1.3 billion in the first 9 months of 2006 (80% from the US).[35] The first generic versions were approved by the US FDA in December 2006, with marketing approval granted to Teva Pharmaceuticals USA and SICOR Pharmaceuticals.[36] In 2018, University of São Paulo and Biolab were granted a patent for an orodispersible form of the drug.[37]
Society and culture
Publication bias
In 1997, ondansetron was the subject of a meta-analysis case study published in the British Medical Journal. Researchers examined 84 trials, with 11,980 people receiving ondansetron, published between 1991 and September 1996. Intravenous ondansetron 4 mg versus placebo was investigated in 16 reports and three further reports which had been duplicated a total of six times. The number needed to treat (NNT) to prevent vomiting within 24 hours was 9.5, with 95% confidence interval 6.9 to 15, in the 16 nonduplicated reports. In the three duplicated reports, the NNT was significantly lower at 3.9 (3.3 to 4.8). When all 25 reports were combined, the apparent NNT improved to 4.9 (4.4 to 5.6). Inclusion of duplicate reports led to a 23% overestimation of ondansetron's antiemetic efficacy.[38]
In addition, the authors found the covert duplication of reports on ondansetron was not easy to detect, because of lack of cross-referencing between papers, and reports containing duplicate findings were cited in eight reviews of the drug.[38] Their analysis was a subject of an editorial in the Journal of the American Medical Association in 1999.[39]
Availability
Ondansetron is a generic drug and is available in many countries under many brand names.[2]
Routes of administration
It can be given by mouth, as a tablet or orally disintegrating tablet, or by injection into a muscle or into a vein.[9]
Research
Psychiatric disorders
A 2006 double-blind, randomized controlled trial indicated ondansetron may have value in the treatment of schizophrenia, as an adjunct to haloperidol. The study found the combination to significantly improve negative schizophrenia symptoms, and people taking both drugs experienced fewer of the adverse effects commonly associated with haloperidol.[40] An earlier, smaller, open-label trial had found ondansetron to be useful in treating antipsychotic-induced tardive dyskinesia in people with schizophrenia, and the study patients also showed significant improvement in the disease's symptoms.[41][42]
Early studies have also examined ondansetron as a possible treatment for psychosis resulting from advanced Parkinson's disease.[43] Its apparent benefits despite a lack of any significant antagonistic properties at dopamine receptors or the 5-HT2A receptor raises interesting questions about the etiology of psychosis.
Substance use
There is tentative evidence that it may be useful in decreasing the desired effects of alcohol.[44] There is also some tentative evidence in those who are addicted to stimulants.[45]
Postanesthetic shivering
Two small, placebo-controlled trials have been conducted to assess the efficacy of ondansetron for postanesthetic shivering, a common occurrence after surgery. Ondansetron was found to be as effective as pethidine (meperidine, Demerol) when given as a single intravenous dose before anesthesia.[46]
References
- "Atossa". MedicinesFAQ. 2022. Archived from the original on 28 August 2022. Retrieved 28 August 2022.
- "Ondansetron international". Drugs.com. 2 September 2020. Archived from the original on 21 February 2014. Retrieved 2 February 2014.
- "Ondansetron Use During Pregnancy". Drugs.com. 3 October 2019. Retrieved 7 September 2020.
- "Zofran Product and Consumer Medicine Information Licence". TGA eBS. Retrieved 28 August 2022.
- "Zofran Product information". Health Canada. 25 April 2012. Retrieved 28 August 2022.
- "Zofran Tablets 4 mg - Summary of Product Characteristics (SmPC)". (emc). 19 January 2022. Retrieved 28 August 2022.
- "Zofran- ondansetron hydrochloride tablet, film coated". DailyMed. 24 June 2020. Retrieved 7 September 2020.
- "List of nationally authorised medicinal products : Active substance: ondansetron :Procedure no.: PSUSA/00002217/202102" (PDF). Ema.europa.eu. Retrieved 5 March 2022.
- "Ondansetron Hydrochloride". The American Society of Health-System Pharmacists. Archived from the original on 3 May 2016. Retrieved 11 February 2017.
- Schnadower D, Finkelstein Y, Freedman SB (January 2015). "Ondansetron and probiotics in the management of pediatric acute gastroenteritis in developed countries". Current Opinion in Gastroenterology. 31 (1): 1–6. doi:10.1097/mog.0000000000000132. PMID 25333367. S2CID 9334264.
- Freedman SB, Ali S, Oleszczuk M, Gouin S, Hartling L (July 2013). "Treatment of acute gastroenteritis in children: an overview of systematic reviews of interventions commonly used in developed countries". Evidence-Based Child Health. 8 (4): 1123–37. doi:10.1002/ebch.1932. PMID 23877938.
- Miloro M, ed. (2012). Peterson's principles of oral and maxillofacial surgery (3rd ed.). Shelton, CT: People's Medical Pub. House-USA. p. 86. ISBN 978-1-60795-111-7. Archived from the original on 1 February 2016.
- FischerJ, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 448. ISBN 9783527607495.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Ondansetron - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- Cooke CE, Mehra IV (March 1994). "Oral ondansetron for preventing nausea and vomiting". American Journal of Hospital Pharmacy. 51 (6): 762–71. PMID 8010314.
- Smith JA, Refuerzo JS, Ramin SM. "Treatment and outcome of nausea and vomiting of pregnancy". UpToDate. Archived from the original on 3 December 2013.
- Carstairs SD (May 2016). "Ondansetron Use in Pregnancy and Birth Defects: A Systematic Review". Obstetrics and Gynecology. 127 (5): 878–83. doi:10.1097/AOG.0000000000001388. PMID 27054939. S2CID 24121438.
- Ramin SM, et al. (Committee on Practice Bulletins-Obstetrics) (January 2018). "ACOG Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy". Obstetrics and Gynecology. 131 (1): e15–30. doi:10.1097/AOG.0000000000002456. PMID 29266076. S2CID 37873516.
- Abell TL, Adams KA, Boles RG, Bousvaros A, Chong SK, Fleisher DR, et al. (April 2008). "Cyclic vomiting syndrome in adults" (PDF). Neurogastroenterology and Motility. 20 (4): 269–84. doi:10.1111/j.1365-2982.2008.01113.x. hdl:2027.42/72300. PMID 18371009. S2CID 8718836.
- Freedman SB, Adler M, Seshadri R, Powell EC (April 2006). "Oral ondansetron for gastroenteritis in a pediatric emergency department". The New England Journal of Medicine. 354 (16): 1698–705. doi:10.1056/NEJMoa055119. PMID 16625009. S2CID 13712069.
- Sturm JJ, Hirsh DA, Schweickert A, Massey R, Simon HK (May 2010). "Ondansetron use in the pediatric emergency department and effects on hospitalization and return rates: are we masking alternative diagnoses?". Annals of Emergency Medicine. 55 (5): 415–22. doi:10.1016/j.annemergmed.2009.11.011. PMID 20031265.
- Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, et al. (October 2014). "A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea". Gut. 63 (10): 1617–1625. doi:10.1136/gutjnl-2013-305989. PMID 24334242.
- Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ (December 1997). "Efficacy, dose-response, and safety of ondansetron in prevention of postoperative nausea and vomiting: a quantitative systematic review of randomized placebo-controlled trials". Anesthesiology. 87 (6): 1277–89. doi:10.1097/00000542-199712000-00004. PMID 9416710. S2CID 8049193.
- US Food and Drug Administration. (2012). FDA Drug Safety Communication: New information regarding QT prolongation with ondansetron (Zofran). Retrieved from "FDA Drug Safety Communication: New information regarding QT prolongation with ondansetron (Zofran)". Food and Drug Administration. Archived from the original on 14 December 2012. Retrieved 29 November 2012.
- Browning KN (October 2015). "Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology". Frontiers in Neuroscience. 9: 413. doi:10.3389/fnins.2015.00413. PMC 4625078. PMID 26578870.
- Schinkel AH, Wagenaar E, Mol CA, van Deemter L (June 1996). "P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs". J Clin Invest. 97 (11): 2517–24. doi:10.1172/JCI118699. PMC 507337. PMID 8647944.
- Kwan C, Bédard D, Frouni I, Gaudette F, Beaudry F, Hamadjida A, Huot P (July 2020). "Pharmacokinetic profile of the selective 5-HT3 receptor antagonist ondansetron in the rat: an original study and a minireview of the behavioural pharmacological literature in the rat". Can J Physiol Pharmacol. 98 (7): 431–440. doi:10.1139/cjpp-2019-0551. PMID 32017606.
- Scott JA, Wood M, Flood P (September 2006). "The pronociceptive effect of ondansetron in the setting of P-glycoprotein inhibition". Anesth Analg. 103 (3): 742–6. doi:10.1213/01.ane.0000228861.80314.22. PMID 16931690.
- US patent 4695578, Coates IH, Bell JA, Humber DC, Ewan GB, "1,2,3,9-tetrahydro-3-imidazol-1-ylmethyl-4H-carbazol-4-ones, composition containing them, and method of using them to treat neuronal 5HT function disturbances", issued 22 September 1987, assigned to Glaxo Group Limited
- US patent 4753789, Tyers MB, Coates IH, Humber DC, Ewan GB, Bell JA, "Method for treating nausea and vomiting", issued 28 June 1988, assigned to Glaxo Group Limited
- US patent 5578628, Tyers MB, Coates IH, Humber DC, Ewan GB, Bell JA, "Medicaments for the treatment of nausea and vomiting", issued 26 November 1996, assigned to Glaxo Group Limited
- "One Year Post-Pediatric Exclusivity Post-marketing Adverse Event Review: Drug Use Data Zofran" (PDF). Memorandum. U.S. Food and Drug Administration. 7 March 2006. Archived (PDF) from the original on 24 September 2015.
- IHS. (2006). Generics firms line up to enter Zofran market. Retrieved from "Generics Firms Line Up to Enter Zofran Market". Archived from the original on 1 February 2014. Retrieved 20 January 2014.
- "FDA Approves First Generic Ondansetron Tablets, Orally Disintegrating Tablets and Oral Solution" (Press release). U.S. Food and Drug Administration. 17 December 2006. Archived from the original on 18 June 2014.
- "Sabia que um remédio para enjoo traz 90% dos royalties que a USP recebe? - Agência USP de Inovação" (in Brazilian Portuguese). Retrieved 6 October 2020.
- Tramèr MR, Reynolds DJ, Moore RA, McQuay HJ (September 1997). "Impact of covert duplicate publication on meta-analysis: a case study". BMJ. 315 (7109): 635–40. doi:10.1136/bmj.315.7109.635. PMC 2127450. PMID 9310564.
- Rennie D (November 1999). "Fair conduct and fair reporting of clinical trials". JAMA. 282 (18): 1766–8. doi:10.1001/jama.282.18.1766. PMID 10568651.
- Zhang ZJ, Kang WH, Li Q, Wang XY, Yao SM, Ma AQ (December 2006). "Beneficial effects of ondansetron as an adjunct to haloperidol for chronic, treatment-resistant schizophrenia: a double-blind, randomized, placebo-controlled study". Schizophrenia Research. 88 (1–3): 102–10. doi:10.1016/j.schres.2006.07.010. PMID 16959472. S2CID 24911372.
- Zullino DF, Eap CB, Voirol P (April 2001). "Ondansetron for tardive dyskinesia". The American Journal of Psychiatry. 158 (4): 657–8. doi:10.1176/appi.ajp.158.4.657-a. PMID 11282718.
- Sirota P, Mosheva T, Shabtay H, Giladi N, Korczyn AD (February 2000). "Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia". The American Journal of Psychiatry. 157 (2): 287–9. doi:10.1176/appi.ajp.157.2.287. PMID 10671405.
- Zoldan J, Friedberg G, Livneh M, Melamed E (July 1995). "Psychosis in advanced Parkinson's disease: treatment with ondansetron, a 5-HT3 receptor antagonist". Neurology. 45 (7): 1305–8. doi:10.1212/WNL.45.7.1305. PMID 7617188. S2CID 45540572.
- Miller PM, Book SW, Stewart SH (2011). "Medical treatment of alcohol dependence: a systematic review". International Journal of Psychiatry in Medicine. 42 (3): 227–66. doi:10.2190/pm.42.3.b. PMC 3632430. PMID 22439295.
- Lee TH, Szabo ST, Fowler JC, Mannelli P, Mangum OB, Beyer WF, et al. (July 2012). "Pharmacologically-mediated reactivation and reconsolidation blockade of the psychostimulant-abuse circuit: a novel treatment strategy". Drug and Alcohol Dependence. 124 (1–2): 11–8. doi:10.1016/j.drugalcdep.2012.01.021. PMC 3500569. PMID 22356892.
- Generali JA, Cada DJ (August 2009). "Ondansetron: postanesthetic shivering" (PDF). Hospital Pharmacy. 44 (8): 670–1. doi:10.1310/hpj4408-670. S2CID 81190955. Archived (PDF) from the original on 10 July 2011.
External links
- "Ondansetron". Drug Information Portal. U.S. National Library of Medicine.