Sumatriptan
Sumatriptan, sold commonly under brand names Imitrex and Treximet among others, is a medication used to treat migraine headaches and cluster headaches.[1] It is taken orally, intranasally, or by subcutaneous injection.[2] Therapeutic effects generally occur within three hours.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Imitrex, Imigran, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | By mouth, subcutaneous injection, nasal spray, transdermal electrophoresis |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 15% (by mouth) / 96% (by subcutaneous injection) |
| Protein binding | 14–21% |
| Metabolism | MAO |
| Elimination half-life | 2.5 hours |
| Excretion | 60% urine; 40% feces |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.130.518 |
| Chemical and physical data | |
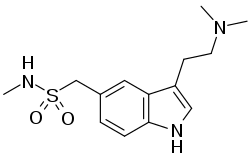
| Formula | C14H21N3O2S |
| Molar mass | 295.40 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Its primary effect as a serotonin 5-HT1B/1D receptor agonist[3] can create common side effects such as chest pressure, fatigue, vomiting, tingling, and vertigo.[2] Serious side effects may include serotonin syndrome, heart attacks, strokes, and seizures.[2] With excessive medication overuse headaches may occur.[2] It is unclear if use during pregnancy or breastfeeding is safe.[4] The mechanism of action not entirely clear.[2] It is in the triptan class of medications.[2]
Sumatriptan was patented in 1982 and approved for medical use in 1991.[5] It is on the World Health Organization's List of Essential Medicines.[6] It is available as a generic medication.[1] In 2020, it was the 111th most commonly prescribed medication in the United States, with more than 5 million prescriptions.[7][8] It is also available as the combination product sumatriptan/naproxen.
Medical uses
Sumatriptan is effective for ending or relieving the intensity of migraine and cluster headaches.[9] It is most effective when taken early after the start of the pain.[9] Injected sumatriptan is more effective than other formulations.[10]
Oral sumatriptan can be used also in the treatment of post-dural puncture headache.[11]
Adverse effects
Overdose of sumatriptan can cause sulfhemoglobinemia, a rare condition in which the blood changes from red to green, due to the integration of sulfur into the hemoglobin molecule.[12] If sumatriptan is discontinued, the condition reverses within a few weeks.
Serious cardiac events, including some that have been fatal, have occurred following the use of sumatriptan injection or tablets. Events reported have included coronary artery vasospasm, transient myocardial ischemia, myocardial infarction, ventricular tachycardia, and ventricular fibrillation.[13]
The most common side effects[14] reported by at least 2% of patients in controlled trials of sumatriptan (25-, 50-, and 100-mg tablets) for migraine are atypical sensations (paresthesias and warm/cold sensations) reported by 4% in the placebo group and 5–6% in the sumatriptan groups, pain and other pressure sensations (including chest pain) reported by 4% in the placebo group and 6–8% in the sumatriptan groups, neurological events (vertigo) reported by less than 1% in the placebo group and less than 1% to 2% in the sumatriptan groups. Malaise/fatigue occurred in less than 1% of the placebo group and 2–3% of the sumatriptan groups. Sleep disturbance occurred in less than 1% in the placebo group to 2% in the sumatriptan group.
Mechanism of action
Sumatriptan is molecularly similar to serotonin (5-HT), and is a 5-HT receptor (types 5-HT1D and 5-HT1B[15]) agonist. Sumatriptan's primary therapeutic effect is related in its inhibition of the release of Calcitonin gene-related peptide (CGRP), likely through its 5-HT1D/1B receptor-agonist action.[16] This has been substantiated by the efficacy of more recently developed CGRP targeting drugs and antibodies developed for the preventive treatment of migraine.[17] How agonism of the 5-HT1D/1B receptors inhibits CGRP release is not fully understood. CGRP is believed to cause sensitization of trigeminal nociceptive neurons, contributing to the pain experienced in migraine.[18]
Sumatriptan is also shown to decrease the activity of the trigeminal nerve, which presumably accounts for sumatriptan's efficacy in treating cluster headaches. The injectable form of the drug has been shown to abort a cluster headache within 30 minutes in 77% of cases.[19]
Pharmacokinetics
Sumatriptan is administered in several forms: tablets, subcutaneous injection, and nasal spray. Oral administration (as succinate salt) has low bioavailability, partly due to presystemic metabolism—some of it gets broken down in the stomach and bloodstream before it reaches the target arteries. A rapid-release tablet formulation with the same bioavailability but a high concentration can achieve therapeutic effects on average 10–15 minutes earlier than other oral forumulations. When injected, sumatriptan is faster-acting (usually within 10 minutes), but the effect lasts for a shorter time. Sumatriptan is metabolised primarily by monoamine oxidase A into 2-{5-[(methylsulfamoyl)methyl]-indole-3-yl}acetic acid, which is then conjugated to glucuronic acid. These metabolites are excreted in the urine and bile. Only about 3% of the active drug may be recovered unchanged.
There is no simple, direct relationship between sumatriptan concentration (pharmacokinetics) per se in the blood and its anti-migraine effect (pharmacodynamics). This paradox has, to some extent, been resolved by comparing the rates of absorption of the various sumatriptan formulations, rather than the absolute amounts of drug that they deliver.[20][21]
History
In 1991, Glaxo received approval for sumatriptan, which was the first available triptan.
In July 2009, the US FDA approved a single-use jet injector formulation of sumatriptan. The device delivers a subcutaneous injection of sumatriptan, without the use of a needle. Autoinjectors with needles have been previously available in Europe and North America.[22]
Phase III studies with an iontophoretic transdermal patch (Zelrix/Zecuity) started in July 2008.[23] This patch uses low voltage controlled by a pre-programmed microchip to deliver a single dose of sumatriptan through the skin within 30 minutes.[24][25] Zecuity was approved by the US FDA in January 2013.[26] Sales of Zecuity have been stopped following reports of skin burns and irritation.[27]
Society and culture
Legal status
In the United States, it is available only by medical prescription. It is available over the counter in many states in Australia. The product requires labelling by a pharmacist and is only available in packs of two without a medical prescription.[28] However, it can be bought over the counter in the UK[29] and Sweden.[30]
In Russia versions of sumatriptan, which are not registered in the National registry of medications, may be regarded as narcotic drugs (derivatives of dimethyltriptamine).[31]
Generics
Glaxo patents for sumatriptan expired in February 2009. At that time, Imitrex sold for about $25 a pill.[32] Par Pharmaceutical then introduced generic versions of sumatriptan injection (sumatriptan succinate injection) 4- and 6-mg starter kits and 4- and 6-mg filled syringe cartridges, and 6-mg vials soon after.[33]
Mylan Laboratories Inc., Ranbaxy Laboratories, Sandoz (a subsidiary of Novartis), Dr. Reddy's Laboratories, and other companies have been producing generic versions of sumatriptan tablets in 25-, 50-, and 100-mg doses. Generic forms of the drug are available in U.S. and European markets after Glaxo's patent protections expired in the respective countries. A nasal spray form of sumatriptan known as AVP-825 has been developed by Avanir and is generically available in some countries.[34]
Controversy
According to the American Headache Society, "Patients frequently state that they have difficulty accessing triptans prescribed to them."[35] In the U.S. triptans cost from $12 to $120 each, and more that 80% of U.S. health insurance plans limit place a limit on the amount of pills available to a patient per month, which has been called "arbitrary and unfair."[36]
References
- British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. p. 474. ISBN 9780857113382.
- "Sumatriptan Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- Syed, Yahiya Y. (January 2016). "Sumatriptan/Naproxen Sodium: A Review in Migraine". Drugs. 76 (1): 111–121. doi:10.1007/s40265-015-0521-8. ISSN 1179-1950. PMID 26628293.
- "Sumatriptan Use During Pregnancy". Drugs.com. Retrieved 3 March 2019.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 531. ISBN 9783527607495.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Sumatriptan - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- Derry, CJ; Derry, S; Moore, RA (28 May 2014). "Sumatriptan (all routes of administration) for acute migraine attacks in adults - overview of Cochrane reviews". The Cochrane Database of Systematic Reviews. 5 (5): CD009108. doi:10.1002/14651858.CD009108.pub2. PMC 6469574. PMID 24865446.
- Dahlöf, Carl G. H. (2001). "Sumatriptan: Pharmacological Basis and Clinical Results". Current Medical Research and Opinion. 17 Suppl 1: s35-45. doi:10.1185/0300799039117010. PMID 12463276. S2CID 2355125. Archived from the original on 17 February 2017. Retrieved 16 July 2016.
- Shaat, Ahmed Mohamed; Abdalgaleil, Mohamed Mahmoud (1 January 2021). "Is theophylline more effective than sumatriptan in the treatment of post-dural puncture headache? A randomized clinical trial". Egyptian Journal of Anaesthesia. 37 (1): 310–316. doi:10.1080/11101849.2021.1949195. ISSN 1110-1849.
- "Patient bleeds dark green blood". BBC News. 8 June 2007. Archived from the original on 5 August 2010. Retrieved 6 March 2010.
- Kelly KM (June 1995). "Cardiac arrest following use of sumatriptan". Neurology. 45 (6): 1211–3. doi:10.1212/wnl.45.6.1211. PMID 7783891. S2CID 35168945.
- "Tablets". fda.gov. Retrieved 19 February 2018.
- Razzaque Z, Heald MA, Pickard JD, et al. (1999). "Vasoconstriction in human isolated middle meningeal arteries: determining the contribution of 5-HT1B- and 5-HT1F-receptor activation". Br J Clin Pharmacol. 47 (1): 75–82. doi:10.1046/j.1365-2125.1999.00851.x. PMC 2014192. PMID 10073743.
- Juhasz, G; Zsombok, T; Jakab, B; Nemeth, J; Szolcsanyi, J; Bagdy, G (26 June 2016). "Sumatriptan Causes Parallel Decrease in Plasma Calcitonin Gene-Related Peptide (CGRP) Concentration and Migraine Headache During Nitroglycerin Induced Migraine Attack". Cephalalgia. 25 (3): 179–183. doi:10.1111/j.1468-2982.2005.00836.x. PMID 15689192. S2CID 13007101.
- Tso, Amy R.; Goadsby, Peter J. (1 August 2017). "Anti-CGRP Monoclonal Antibodies: the Next Era of Migraine Prevention?". Current Treatment Options in Neurology. 19 (8): 27. doi:10.1007/s11940-017-0463-4. ISSN 1092-8480. PMC 5486583. PMID 28653227.
- Giniatullin, Rashid; Nistri, Andrea; Fabbretti, Elsa (1 February 2008). "Molecular Mechanisms of Sensitization of Pain-transducing P2X3 Receptors by the Migraine Mediators CGRP and NGF". Molecular Neurobiology. 37 (1): 83–90. doi:10.1007/s12035-008-8020-5. ISSN 0893-7648. PMID 18459072. S2CID 25689799.
- Sumatriptan Cluster Headache Study Group (1991). "Treatment of acute cluster headache with sumatriptan. The Sumatriptan Cluster Headache Study Group". N Engl J Med. 325 (5): 322–6. doi:10.1056/NEJM199108013250505. PMID 1647496.
- Fox, A. W. (2004). "Onset of effect of 5-HT1B/1D agonists: a model with pharmacokinetic validation". Headache. 44 (2): 142–147. doi:10.1111/j.1526-4610.2004.04030.x. PMID 14756852. S2CID 25587940.
- Freidank-Mueschenborn, E.; Fox, A. (2005). "Resolution of concentration-response differences in onset of effect between subcutaneous and oral sumatriptan". Headache. 45 (6): 632–637. doi:10.1111/j.1526-4610.2005.05129a.x. PMID 15953294. S2CID 20755695.
- Brandes, J.; Cady, R.; Freitag, F.; Smith, T.; Chandler, P.; Fox, A.; Linn, L.; Farr, S. (2009). "Needle-free subcutaneous sumatriptan (Sumavel DosePro): bioequivalence and ease of use". Headache. 49 (10): 1435–1444. doi:10.1111/j.1526-4610.2009.01530.x. PMID 19849720. S2CID 25696109.
- Clinical trial number NCT00724815 for "The Efficacy and Tolerability of NP101 Patch in the Treatment of Acute Migraine (NP101-007)" at ClinicalTrials.gov
- "SmartRelief -electronically assisted drug delivery (iontophoresis)". nupathe.com. Archived from the original on 7 January 2016. Retrieved 19 February 2018.
- Pierce, M; Marbury, T; O'Neill, C; Siegel, S; Du, W; Sebree, T (2009). "Zelrix: a novel transdermal formulation of sumatriptan". Headache. 49 (6): 817–25. doi:10.1111/j.1526-4610.2009.01437.x. PMID 19438727. S2CID 205683188.
- "Zecuity Approved by the FDA for the Acute Treatment of Migraine". nupathe.com. Archived from the original on 7 January 2016. Retrieved 19 February 2018.
- "Teva pulls migraine patch Zecuity on reports of burning, scarring | FiercePharma". www.fiercepharma.com. 13 June 2016. Archived from the original on 21 March 2017. Retrieved 10 April 2017.
- "Poisons Standard June 2017". 18 May 2017. Archived from the original on 31 July 2017. Retrieved 22 July 2017.
- "Press release: First Over The Counter (OTC) migraine pill made available". Medicines and Healthcare Products Regulatory Agency. Archived from the original on 5 December 2014. Retrieved 28 January 2015.
- European Medicines Agency (23 November 2011). "Assessment Report: Sumatriptan Galpharm 50 mg Tablets" (PDF). European Medicines Agency. p. 20. Archived (PDF) from the original on 7 January 2016. Retrieved 28 January 2015.
- "Постановление Правительства РФ от 30 июня 1998 г. N 681 "Об утверждении перечня наркотических средств, психотропных веществ и их прекурсоров, подлежащих контролю в Российской Федерации" (с изменениями и дополнениями)" (in Russian). Гарант. Retrieved 28 April 2019.
ДМТ (диметилтриптамин) и его производные, за исключением производных, включенных в качестве самостоятельных позиций в перечень
- "GlaxoSmithKline sets out to dupe migraine sufferers with Treximet smoke and mirrors". Community Catalyst. 24 April 2008. Retrieved 22 March 2019.
- "Par Pharmaceutical begins shipment of sumatriptan injection". Par Pharmaceutical. 6 November 2008. Archived from the original on 10 December 2008. Retrieved 25 November 2008.
- LaMattina, John (2 March 2015). "If You 'Want To Make A Good Drug Great' Cost Must Be Factored In". Forbes. Archived from the original on 14 February 2017. Retrieved 13 February 2017.
- Minen, M. T., Lindberg, K., Langford, A., Loder, E. (2017), "Variation in Prescription Drug Coverage for Triptans: Analysis of Insurance Formularies", Headache: The Journal of Head and Face Pain, Wiley, 57 (8): 1243–1251, doi:10.1111/head.13134, PMID 28691382, S2CID 42239120
- Eve Bender (7 September 2017), "Practice Matters Wide Variation in Triptan Coverage Across Commercial and Government Health Plans", Neurology Today, American Academy of Neurology, 17 (17): 7, doi:10.1097/01.NT.0000524839.20893.03, S2CID 80167113, retrieved 26 June 2022
External links
- "Sumatriptan". Drug Information Portal. U.S. National Library of Medicine.