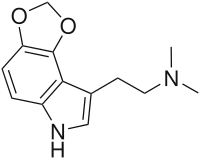
4,5-MDO-DMT
4,5-MDO-DMT, or 4,5-methylenedioxy-N,N-dimethyltryptamine, is a lesser-known psychedelic drug. It is the 4,5-methylenedioxy analog of DMT. 4,5-MDO-DMT was first synthesized by Alexander Shulgin, though in his book TiHKAL it was not tested to determine its psychoactive effects. 4,5-MDO-DMT has been the subject of limited subsequent testing; with behavioral disruption studies performed in male rats indicating that its hallucinogenic potency is less than that of 4,5-MDO-DiPT but greater than that of 5,6-MDO-DiPT.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-(2H,6H-[1,3]Dioxolo[4,5-e]indol-8-yl)-N,N-dimethylethan-1-amine | |
| Other names
4,5-Methylenedioxy-N,N-dimethyltryptamine | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C14H16N2O2 |
| Molar mass | 232.28 |
| Melting point | 93–95 °C (199–203 °F; 366–368 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Kline, Toni B.; Benington, Frederick; Morin, Richard D.; Beaton, John M. (August 1982). "Structure-activity relationships in potentially hallucinogenic N,N-dialkyltryptamines substituted in the benzene moiety". Journal of Medicinal Chemistry. 25 (8): 908–913. doi:10.1021/jm00350a005. PMID 7120280.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.