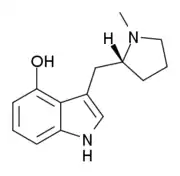
4-HO-MPMI
4-HO-MPMI (also known as 4-Hydroxy-N-methyl-(α,N-trimethylene)-tryptamine or lucigenol) is a tryptamine derivative that is a psychedelic drug. It was developed by the team led by David Nichols from Purdue University in the late 1990s. This compound produces hallucinogen-appropriate responding in animal tests with a similar potency to the amphetamine-derived psychedelic DOI, and has two enantiomers, with only the (R)-enantiomer being active.[1]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H17N2O |
| Molar mass | 229.303 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
The binding affinity for 5-HT2A receptor is 13 ± 2 nM (Ki [125I]DOI). It is reported at doses starting at 0.5 mg and 1.0-1.5 mg seem to be psychedelic doses. The duration it is reported between six and eight hours. The effects, still not too documented, are OEV/CEV, sedation and anxiety.[2]
See also
References
- Gerasimov M, Marona-Lewicka D, Kurrasch-Orbaugh DM, Qandil AM, Nichols DE (1999). "Further studies on oxygenated tryptamines with LSD-like activity incorporating a chiral pyrrolidine moiety into the side chain". Journal of Medicinal Chemistry. 42 (20): 4257–4263. CiteSeerX 10.1.1.690.4941. doi:10.1021/jm990325u. PMID 10514296.
- "Lucigenol Info - Hip Forums". Archived from the original on 2011-07-11. Retrieved 2010-08-02.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.