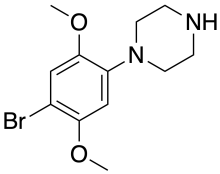
2C-B-PP
2,5-dimethoxy-4-bromophenylpiperazine (2C-B-PP) is a drug of the phenylpiperazine class. It acts as an agonist at serotonin receptors, and in studies on rats substituted for the psychedelic amphetamine derivative DOM with around 1/10 the potency but similar rates of stimulus-appropriate responding at the highest dose.[1][2]: 867–868
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H17BrN2O2 |
| Molar mass | 301.184 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Lyon RA, Titeler M, McKenney JD, Magee PS, Glennon RA (May 1986). "Synthesis and evaluation of phenyl- and benzoylpiperazines as potential serotonergic agents". Journal of Medicinal Chemistry. 29 (5): 630–4. doi:10.1021/jm00155a008. PMID 3701781.
- Trachsel D, Lehmann D, Enzensperger C (2013). Phenethylamine: Von der Struktur zur Funktion. Nachtschatten Verlag AG. ISBN 978-3-03788-700-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.