Cinepazet
Cinepazet is a vasodilator and is an ethyl ester derivative of cinepazic acid.[1]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.738 |
| Chemical and physical data | |
| Formula | C20H28N2O6 |
| Molar mass | 392.452 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.172 g/cm3 |
SMILES
| |
InChI
| |
Synthesis
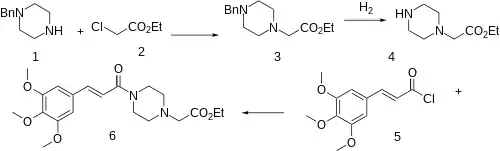
The alkylation between benzylpiperazine (1) and ethyl chloroacetate (2) gives ethyl 2-(4-benzylpiperazin-1-yl)acetate [23173-76-4] (3). The benzyl protecting group is easily cleaved by catalytic hydrogenation giving ethyl 2-(piperazin-1-yl)acetate [40004-08-8] (4). The Schotten-Baumann reaction with 3,4,5-trimethoxycinnamoyl chloride [4521-61-3] (5) gives the amide, and hence completing the synthesis of Cinepazet (6).
References
- Morton IK, Hall JM (1999). "Cinepazet". Concise Dictionary of Pharmacological Agents : Properties and Synonyms. Dordrecht: Springer Netherlands. p. 77. ISBN 9789401144391.
- Raynaud, G.; Pourias, B.; Fauran, F.; Turin, M.; 1977, U.S. Patent 4,029,650 (1977 to Delalande S. A.).
- GB1168108 idem Claude Fauran, Gerard Huguet, Guy Raynaud, Bernard Pourrias, Michel Turin, U.S. Patent 3,590,034 (1971 to Delalande Sa).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.