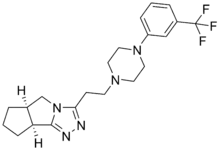
Lorpiprazole
Lorpiprazole (INN) (brand name Normarex) is a marketed anxiolytic drug of the phenylpiperazine group.[1][2][3] It has been described as a serotonin antagonist and reuptake inhibitor (SARI) in the same group as trazodone, nefazodone, and etoperidone.[3]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H26F3N5 |
| Molar mass | 405.469 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Synthesis
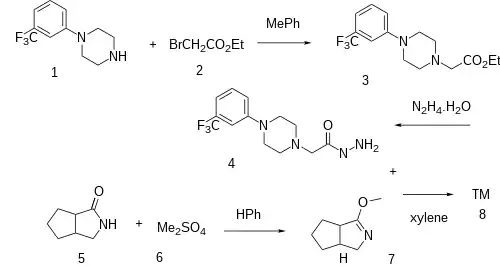
The alkylation between TFMPP (1) and ethyl bromoacetate [105-36-2] (2) gives ethyl 2-[4-[3-(trifluoromethyl)phenyl]piperazin-1-yl]acetate, CID:22199202 (3). Hydrazinolysis of the ester with hydrazine leads to 2-[4-[3-(Trifluoromethyl)phenyl]piperazin-1-yl]acetohydrazide, CID:63572278 (4).
The reaction of 2-oxo-3-azabicyclo(3,3,0)octane [56593-76-1] (5) with Dimethylsulfate [77-78-1] (6) gives the enol ether and hence 2-methoxy-3-azabicyclo(3,3,-0)oct-2-ene, CID:13748840 (7).
Convergent synthesis completed the synthesis of Lorpiprazole (8).
See also
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 742–. ISBN 978-1-4757-2085-3.
- Negwer M, Scharnow H (2001). Organic-Chemical Drugs and Their Synonyms. Vol. 1–6 (8th ed.). Weinheim: Wiley-VCH. ISBN 3-527-30247-6.
- Fagiolini A, Comandini A, Catena Dell'Osso M, Kasper S (December 2012). "Rediscovering trazodone for the treatment of major depressive disorder". CNS Drugs. 26 (12): 1033–49. doi:10.1007/s40263-012-0010-5. PMC 3693429. PMID 23192413.
- Michel Wierzbicki, et al. EP 0199641 (1988 to Adir Et Compagnie); CA, 107,23343
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|