Imepitoin
Imepitoin (INN), sold under the brand name Pexion, is an anticonvulsant which is used in veterinary medicine in Europe to treat epilepsy in dogs.[1][2][3][4] It was recently approved in the United States.[2][3][4] The drug also has anxiolytic effects.[1][2] It was originally developed to treat epilepsy in humans, but clinical trials were terminated upon findings of unfavorable metabolic differences in smokers and non-smokers.[1][2]
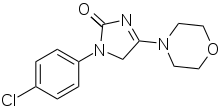 | |
| Clinical data | |
|---|---|
| Trade names | Pexion |
| Other names | AWD 131-138; ELB-138 |
| Routes of administration | Oral |
| ATCvet code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.751 |
| Chemical and physical data | |
| Formula | C13H14ClN3O2 |
| Molar mass | 279.72 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Imepitoin acts as a low-affinity (4,350–5,140 nM; relative to Ki = 6.8 nM for diazepam and Ki = 1.7 nM for clonazepam) partial agonist of the benzodiazepine site of the GABAA receptor (up to 12–21% of the maximal potentiation of diazepam, a full agonist of this site).[1] It is the first partial agonist to be approved for the treatment of epilepsy.[1] The drug also dose-dependently blocks voltage-gated calcium channels.[3] It is not a benzodiazepine; instead, it is an imidazolone, and bears some structural similarities to hydantoin anticonvulsants like ethotoin and phenytoin.[1]
See also
- Separation anxiety in dogs § Benzodiazepine treatment
References
- Rundfeldt C, Löscher W (2014). "The pharmacology of imepitoin: the first partial benzodiazepine receptor agonist developed for the treatment of epilepsy". CNS Drugs. 28 (1): 29–43. doi:10.1007/s40263-013-0129-z. PMID 24357084. S2CID 31627280.
- Sean Sanders (29 April 2015). Seizures in Dogs and Cats. Wiley. pp. 209–. ISBN 978-1-118-68970-7.
- Curtis W. Dewey; Ronaldo C. da Costa (8 September 2015). Practical Guide to Canine and Feline Neurology. Wiley. pp. 806–. ISBN 978-1-119-06204-2.
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
| Alcohols |
|
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
| Calcium |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium |
| ||||||||||||||||||||||||
| Sodium |
| ||||||||||||||||||||||||
| Chloride |
| ||||||||||||||||||||||||
| Others |
| ||||||||||||||||||||||||
See also: Receptor/signaling modulators • Transient receptor potential channel modulators | |||||||||||||||||||||||||