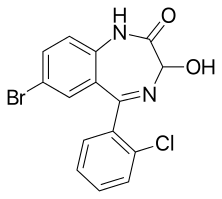
3-Hydroxyphenazepam
3-Hydroxyphenazepam is a benzodiazepine with hypnotic, sedative, anxiolytic, and anticonvulsant properties.[1] It is an active metabolite of phenazepam,[1][2] as well as the active metabolite of the benzodiazepine prodrug cinazepam.[3] Relative to phenazepam, 3-hydroxyphenazepam has diminished myorelaxant properties, but is about equivalent in most other regards.[1] Like other benzodiazepines, 3-hydroxyphenazepam behaves as a positive allosteric modulator of the benzodiazepine site of the GABAA receptor with an EC50 value of 10.3 nM.[4][5][6] It has been sold online as a designer drug.[7][8][9][10]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C15H10BrClN2O2 |
| Molar mass | 365.61 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
- Lorazepam, licensed medication
- Nifoxipam
- Nitemazepam
References
- Artur Viktorovich Valʹdman (31 May 1986). Drug dependence and emotional behavior: neurophysiological and neurochemical approaches. Consultants Bureau. ISBN 978-0-306-10984-3.
- Lukasz Komsta; Monika Waksmundzka-Hajnos; Joseph Sherma (20 December 2013). Thin Layer Chromatography in Drug Analysis. CRC Press. pp. 299–. ISBN 978-1-4665-0715-9.
- Schukin SI, Zinkovsky VG, Zhuk OV (2011). "Elimination kinetics of the novel prodrug cinazepam possessing psychotropic activity in mice" (PDF). Pharmacol Rep. 63 (5): 1093–100. doi:10.1016/s1734-1140(11)70628-4. PMID 22180351.
- "Phenazepam Pre-Review Report" (PDF). World Health Organization (WHO). November 2015.
- Kopanitsa, M. V.; Zbarska, S. M.; Boychuk, Ya. A.; Krishtal, O. A. (2000). "Modulation of GABA-activated currents by phenazepam and its metabolites in isolated rat purkinje neurons". Neurophysiology. 32 (3): 192. doi:10.1007/BF02506568. ISSN 0090-2977. S2CID 32313668.
- Golovenko, N. Ya; Larionov, V. B. (2014). "Pharmacodynamical and Neuroreceptor Analysis of the Permeability of the Blood-Brain Barrier for Derivatives of 1,4-Benzodiazepine". Neurophysiology. 46 (3): 199–205. doi:10.1007/s11062-014-9429-2. ISSN 0090-2977. S2CID 33732669.
- "3-hydroxyphenazepam". New Synthetic Drugs Database. Archived from the original on 2016-09-28. Retrieved 2016-05-30.
- Madeleine Pettersson Bergstrand; Anders Helander; Therese Hansson; Olof Beck (2016). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. 9 (4): 640–645. doi:10.1002/dta.2003. PMID 27366870.
- Moosmann, Bjoern; Bisel, Philippe; Franz, Florian; Huppertz, Laura M.; Auwärter, Volker (2016). "Characterization and in vitro phase I microsomal metabolism of designer benzodiazepines – an update comprising adinazolam, cloniprazepam, fonazepam, 3-hydroxyphenazepam, metizolam, and nitrazolam". Journal of Mass Spectrometry. 51 (11): 1080–1089. Bibcode:2016JMSp...51.1080M. doi:10.1002/jms.3840. ISSN 1096-9888. PMID 27535017.
- Manchester, Kieran R.; Maskell, Peter D.; Waters, Laura (2018). "Experimental versus theoretical log D7.4, pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances" (PDF). Drug Testing and Analysis. 10 (8): 1258–1269. doi:10.1002/dta.2387. ISSN 1942-7611. PMID 29582576.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.