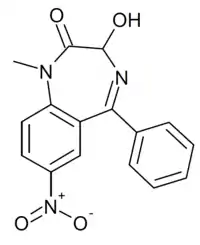
Nitemazepam
Nitemazepam (or 3-hydroxynimetazepam) is a benzodiazepine derivative which was first synthesised in the 1970s but was never marketed. It is the 7-nitro instead of 7-chloro analogue of temazepam, and also the 3-hydroxy derivative of nimetazepam, and an active metabolite. It has in more recent years been sold as a designer drug, first being definitively identified in Europe in 2017. It is metabolized to 7-aminonitemazepam, nimetazepam, 3-hydroxynitemazepam, temazepam, and nimetazepam glucuronide.[1][2]
Not to be confused with nimetazepam or temazepam.
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H13N3O4 |
| Molar mass | 311.297 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Zawilska JB, Wojcieszak J (July 2019). "An expanding world of new psychoactive substances-designer benzodiazepines". Neurotoxicology. 73: 8–16. doi:10.1016/j.neuro.2019.02.015. PMID 30802466.
- Moosmann B, Auwärter V (2018). "Designer Benzodiazepines: Another Class of New Psychoactive Substances.". In Maurer H, Brandt S (eds.). New Psychoactive Substances. Handbook of Experimental Pharmacology. Handbook of Experimental Pharmacology. Vol. 252. pp. 383–410. doi:10.1007/164_2018_154. ISBN 978-3-030-10560-0. PMID 30367253.
| Alcohols |
|
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.