Bromazolam
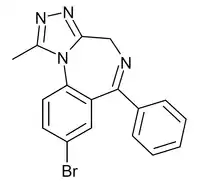
Bromazolam (XLI-268) is a triazolobenzodiazepine (TBZD) which was first synthesised in 1976, but was never marketed.[1] It has subsequently been sold as a designer drug, first being definitively identified by the EMCDDA in Sweden in 2016.[2] It is the bromo instead of chloro analogue of alprazolam and has similar sedative and anxiolytic effects to it and other benzodiazepines.[3][4] Bromazolam is a non subtype selective agonist at the benzodiazepine site of GABAA receptors, with a binding affinity of 2.81nM at the α1 subtype, 0.69nM at α2 and 0.62nM at α5.[5]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H13BrN4 |
| Molar mass | 353.223 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Manchester KR, Lomas EC, Waters L, Dempsey FC, Maskell PD (January 2018). "The emergence of new psychoactive substance (NPS) benzodiazepines: A review". Drug Testing and Analysis. 10 (1): 37–53. doi:10.1002/dta.2211. PMID 28471096.
- "Europol 2016 Annual Report on the implementation of Council Decision 2005/387/JHA. EMCDDA" (PDF). European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) and European Union Agency for Law Enforcement Cooperation (Europol). 2017.
- Waters L, Manchester KR, Maskell PD, Haegeman C, Haider S (May 2018). "The use of a quantitative structure-activity relationship (QSAR) model to predict GABA-A receptor binding of newly emerging benzodiazepines" (PDF). Science & Justice. 58 (3): 219–225. doi:10.1016/j.scijus.2017.12.004. PMID 29685303.
- Zawilska JB, Wojcieszak J (July 2019). "An expanding world of new psychoactive substances-designer benzodiazepines". Neurotoxicology. 73: 8–16. doi:10.1016/j.neuro.2019.02.015. PMID 30802466.
- Clayton T, Poe MM, Rallapalli S, Biawat P, Savić MM, Rowlett JK, Gallos G, Emala CW, Kaczorowski CC, Stafford DC, Arnold LA, Cook JM (2015). "A Review of the Updated Pharmacophore for the Alpha 5 GABA(A) Benzodiazepine Receptor Model". International Journal of Medicinal Chemistry. 2015: 430248. doi:10.1155/2015/430248. PMC 4657098. PMID 26682068.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.