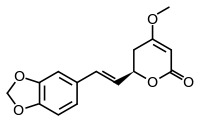
Methysticin
Methysticin is one of the six major kavalactones found in the kava plant.[1] Research suggests that methysticin and the related compound dihydromethysticin have CYP1A1 inducing effects which may be responsible for their toxicity.[2]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(6R)-6-[(E)-2-(2H-1,3-Benzodioxol-5-yl)ethen-1-yl]-4-methoxy-5,6-dihydro-2H-pyran-2-one | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H14O5 |
| Molar mass | 274.272 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Toxicity
Methysticin induces the function of the hepatic enzyme CYP1A1. This enzyme is involved in the toxification of benzo[a]pyrene into (+)-benzo[a]pyrene-7,8-dihydrodiol-9,10-epoxide, a highly carcinogenic substance. Another related compound is dihydromethysticin, which also induces the function of CYP1A1.[2][3][4] No report so far has described enhancement of CYP1A1 expression in animals or humans in vivo from any constituent of kava.[5]
References
- Malani, Joji (2002-12-03). "Evaluation of the effects of Kava on the Liver" (PDF). Fiji School of Medicine. Archived from the original (PDF) on 2009-03-20. Retrieved 2009-09-04.
- Li Y, Mei H, Wu Q, Zhang S, Fang JL, Shi L, Guo L (Dec 2011). "Methysticin and 7,8-dihydromethysticin are two major kavalactones in kava extract to induce CYP1A1". Toxicological Sciences. 124 (2): 388–99. doi:10.1093/toxsci/kfr235. PMC 5736320. PMID 21908763.
- Beresford, AP (1993). "CYP1A1: friend or foe?". Drug Metab Rev. 25 (4): 503–17. doi:10.3109/03602539308993984. PMID 8313840.
- Uno, S; Dalton TP; Durkenne S; Curran CP (2004). "Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation". Molecular Pharmacology. 65 (5): 1225–37. doi:10.1124/mol.65.5.1225. PMID 15102951. S2CID 24627183.
- Yamazaki, Yuko; Hashida, Hiroko; Arita, Anna; Hamaguchi, Keiko; Shimura, Fumio (2008). "High dose of commercial products of kava (Piper methysticum) markedly enhanced hepatic cytochrome P450 1A1 mRNA expression with liver enlargement in rats". Food and Chemical Toxicology. 46 (12): 3732–3738. doi:10.1016/j.fct.2008.09.052. ISSN 0278-6915. PMID 18930106.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.