Elfazepam
Elfazepam[1] is a drug which is a benzodiazepine derivative.[2] Presumably it has sedative and anxiolytic actions like those of other benzodiazepines.
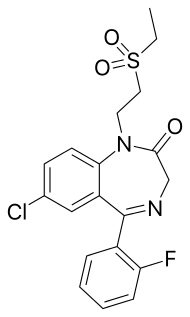 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C19H18ClFN2O3S |
| Molar mass | 408.87 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Orexigenic properties in animals.[3][4] The mechanism for increasing feed intake is not clear and has been subject of investigation.[5] It has been found that elfazepam suppresses gastric acid secretion.[6]
Synthesis

Elfazepam synthesis: U.S. Patent 4,010,154
Benzophenone derivative 1 is reacted with a glycine equivalent masked as an oxazolidine-2,5-dione 2 to give the final product 3 Elfazepam.
References
- U.S. Patent 4,010,154
- Psychotropics.dk
- Baile CA, McLaughlin CL (November 1979). "A review of the behavioral and physiological responses to elfazepam, a chemical feed intake stimulant". Journal of Animal Science. 49 (5): 1371–95. doi:10.2527/jas1979.4951371x. PMID 396294.
- Baile CA, Naylor J, McLaughlin CL, Catanzaro CA (August 1981). "Endotoxin-elicited fever and anorexia and elfazepam-stimulated feeding in sheep". Physiology & Behavior. 27 (2): 271–7. doi:10.1016/0031-9384(81)90269-9. PMID 7029576. S2CID 12408796.
- Keim DA, Baile CA, Bolton JR, Wangsness PJ, Della Fera MA (January 1979). "Abomasal function following injections of elfazepam and 9-aza-cannabinol". Pharmacology, Biochemistry, and Behavior. 10 (1): 63–70. doi:10.1016/0091-3057(79)90170-9. PMID 35793. S2CID 915512.
- Van Den Broek GW, Robertson J, Keim DA, Baile CA (July 1979). "Feeding and depression of abomasal secretion in sheep elicited by elfazepam and 9-aza-cannabinol". Pharmacology, Biochemistry, and Behavior. 11 (1): 51–6. doi:10.1016/0091-3057(79)90296-x. PMID 493298. S2CID 33152825.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.