Etazolate
Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties.[1][2][3] It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site,[4][5][6][7] as an adenosine antagonist of the A1 and A2 subtypes,[8] and as a phosphodiesterase inhibitor selective for the PDE4 isoform.[9][10][11] It is currently in clinical trials for the treatment of Alzheimer's disease.[12]
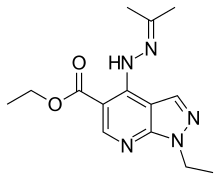 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C14H19N5O2 |
| Molar mass | 289.339 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
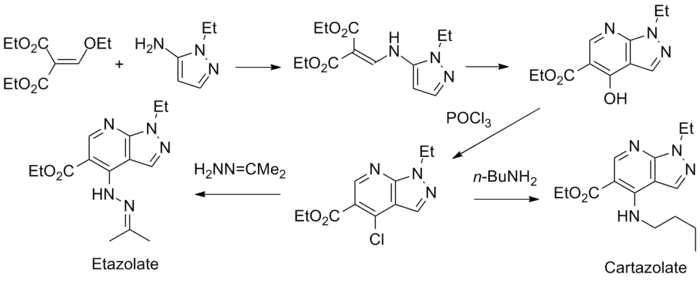
Cartazolate and Etazolate synthesis[13]
See also
References
- Hall, Judith A.; Morton, Ian (1999). Concise dictionary of pharmacological agents: properties and synonyms. Kluwer Academic. ISBN 0-7514-0499-3.
- Williams M (May 1983). "Anxioselective anxiolytics". Journal of Medicinal Chemistry. 26 (5): 619–28. doi:10.1021/jm00359a001. PMID 6132997.
- Williams M, Risley EA (February 1979). "Enhancement of the binding of 3H-diazepam to rat brain membranes in vitro by SQ 20009, A novel anxiolytic, gamma-aminobutyric acid (GABA) and muscimol". Life Sciences. 24 (9): 833–41. doi:10.1016/0024-3205(79)90367-9. PMID 449623.
- Zezula J, Slany A, Sieghart W (April 1996). "Interaction of allosteric ligands with GABAA receptors containing one, two, or three different subunits". European Journal of Pharmacology. 301 (1–3): 207–14. doi:10.1016/0014-2999(96)00066-0. PMID 8773466.
- Remington, Gary; Baskys, Andrius (1996). Brain mechanisms and psychotropic drugs. Boca Raton: CRC Press. ISBN 0-8493-8386-2.
- M. Mishina; Kurachi, Yoshihisa (2000). Pharmacology of ionic channel function: activators and inhibitors. Berlin: Springer. ISBN 3-540-66127-1.
- Progress in Drug Research / Volume 31 (Progress in Drug Research). Boston: Birkhauser. 1987. p. 526. ISBN 3-7643-1837-6.
- Williams M, Jarvis MF (February 1988). "Adenosine antagonists as potential therapeutic agents". Pharmacology Biochemistry and Behavior. 29 (2): 433–41. doi:10.1016/0091-3057(88)90182-7. PMID 3283781. S2CID 35635747.
- Chasin M, Harris DN, Phillips MB, Hess SM (September 1972). "1-Ethyl-4-(isopropylidenehydrazino)-1H-pyrazolo-(3,4-b)-pyridine-5-carboxylic acid, ethyl ester, hydrochloride (SQ 20009)--a potent new inhibitor of cyclic 3',5'-nucleotide phosphodiesterases". Biochemical Pharmacology. 21 (18): 2443–50. doi:10.1016/0006-2952(72)90414-5. PMID 4345859.
- Wang P, Myers JG, Wu P, Cheewatrakoolpong B, Egan RW, Billah MM (May 1997). "Expression, purification, and characterization of human cAMP-specific phosphodiesterase (PDE4) subtypes A, B, C, and D". Biochemical and Biophysical Research Communications. 234 (2): 320–4. doi:10.1006/bbrc.1997.6636. PMID 9177268.
- Gresele, Paolo; Gresele, P. (2002). Platelets in thrombotic and non-thrombotic disorders: pathophysiology, pharmacology and therapeutics. Cambridge, UK: Cambridge University Press. ISBN 0-521-80261-X.
- "Pipeline | ExonHit". Archived from the original on 2011-01-11.
- DE 2123318, Hoehn, Hans & Denzel, Theodor, "Aminoderivate von Pyrazopyridincarbonsäuren, deren Estern und Salzen, Verfahren zu ihrer Herstellung und ihre Verwendung [Amino derivatives of pyrazolopyridinecarboxylic acids, their esters and salts, processes for their preparation and their use]", published 1971-12-09, assigned to E.R. Squibb & Sons Inc.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.