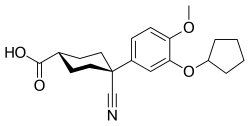
Cilomilast
Cilomilast (INN,[1] codenamed SB-207,499, proposed trade name Ariflo) is a drug which was developed for the treatment of respiratory disorders such as asthma and chronic obstructive pulmonary disease (COPD). It is orally active and acts as a selective phosphodiesterase-4 inhibitor.[2]
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth (tablets) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H25NO4 |
| Molar mass | 343.423 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Phosphodiesterase (PDE) inhibitors, such as theophylline, have been used to treat COPD for centuries; however, the clinical benefits of these agents have never been shown to outweigh the risks of their numerous adverse effects. Four clinical trials were identified evaluating the efficacy of cilomilast, the usual randomized, double-blind, and placebo-controlled protocols were used. It showed reasonable efficacy for treating COPD, but side effects were problematic and it is unclear whether cilomalast will be marketed, or merely used in the development of newer drugs.[3][4]
Cilomilast is a second-generation PDE4 inhibitor with anti-inflammatory effects that target bronchoconstriction, mucus hypersecretion, and airway remodeling associated with COPD.
History
GlaxoSmithKline (GSK) filed for drug approval with the U.S. FDA at the end of 2002 and in January 2003 with the European Medicines Evaluation Agency (EMEA). In October 2003 the FDA issued an approvable letter for use of cilomilast in maintenance of lung function in COPD patients poorly responsive to salbutamol, despite an earlier decision by the FDA advisory panel to reject approval. The rejection was based on concerns over the efficacy of the agent, as well as gastrointestinal side effects.[5] Before issuing final approval, however, the FDA requested additional efficacy and safety data. The development of the drug was finally abandoned by GSK.[6]
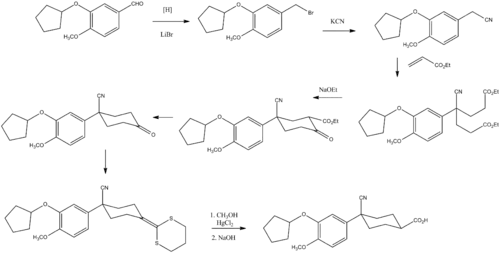
Synthesis
References
- "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). Recommended International Nonproprietary Names (Rec. INN): List 44. World Health Organization. p. 188. Retrieved 3 October 2016.
- Martina SD, Ismail MS, Vesta KS (October 2006). "Cilomilast: orally active selective phosphodiesterase-4 inhibitor for treatment of chronic obstructive pulmonary disease". The Annals of Pharmacotherapy. 40 (10): 1822–8. doi:10.1345/aph.1H049. PMID 16985092. S2CID 23187911.
- Torphy TJ, Barnette MS, Underwood DC, Griswold DE, Christensen SB, Murdoch RD, et al. (1999). "Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic". Pulmonary Pharmacology & Therapeutics. 12 (2): 131–5. doi:10.1006/pupt.1999.0181. PMID 10373396.
- Ochiai H, Ohtani T, Ishida A, Kusumi K, Kato M, Kohno H, et al. (January 2004). "Highly potent PDE4 inhibitors with therapeutic potential". Bioorganic & Medicinal Chemistry Letters. 14 (1): 207–10. doi:10.1016/j.bmcl.2003.09.087. PMID 14684329.
- "FDA Panel Rejects GSK's Ariflo". www.thepharmaletter.com. September 15, 2003. Retrieved 3 October 2016.
- Francis SH, Conti M, Houslay MD, eds. (2011). Phosphodiesterases as drug targets. Berlin, Heidelberg: Springer-Verlag. p. 89. ISBN 978-3-642-17968-6.
- Christensen SB, Guider A, Forster CJ, Gleason JG, Bender PE, Karpinski JM, et al. (March 1998). "1,4-Cyclohexanecarboxylates: potent and selective inhibitors of phosophodiesterase 4 for the treatment of asthma". Journal of Medicinal Chemistry. 41 (6): 821–35. doi:10.1021/jm970090r. PMID 9526558.