BRL-50481
BRL-50481 is a drug developed by GlaxoSmithKline which is the first compound that acts as a phosphodiesterase inhibitor selective for the PDE7 family.[1] PDE7 activity is encoded by two genes, PDE7A and PDE7B. BRL-50481 actually shows about an 80-fold preference for the PDE7A subtype, for which it was developed, over PDE7B.[2] BRL-50481 has been shown to increase mineralisation activity in osteoblasts, suggesting a potential role for PDE7 inhibitors in the treatment of osteoporosis.[3]
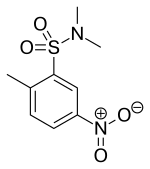 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C9H12N2O4S |
| Molar mass | 244.27 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- Smith SJ, Cieslinski LB, Newton R, Donnelly LE, Fenwick PS, Nicholson AG, et al. (December 2004). "Discovery of BRL 50481 [3-(N,N-dimethylsulfonamido)-4-methyl-nitrobenzene], a selective inhibitor of phosphodiesterase 7: in vitro studies in human monocytes, lung macrophages, and CD8+ T-lymphocytes" (PDF). Molecular Pharmacology. 66 (6): 1679–89. doi:10.1124/mol.104.002246. PMID 15371556. S2CID 9491524.
- Alaamery MA, Wyman AR, Ivey FD, Allain C, Demirbas D, Wang L, et al. (April 2010). "New classes of PDE7 inhibitors identified by a fission yeast-based HTS". Journal of Biomolecular Screening. 15 (4): 359–67. doi:10.1177/1087057110362100. PMC 2854023. PMID 20228279.
- Pekkinen M, Ahlström ME, Riehle U, Huttunen MM, Lamberg-Allardt CJ (July 2008). "Effects of phosphodiesterase 7 inhibition by RNA interference on the gene expression and differentiation of human mesenchymal stem cell-derived osteoblasts". Bone. 43 (1): 84–91. doi:10.1016/j.bone.2008.02.021. PMID 18420479.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.