Filaminast
Filaminast (code name WAY-PDA 641)[1] was a drug candidate developed by Wyeth-Ayerst.[2]: Table 2 It is a phosphodiesterase 4 inhibitor (PDE4 inhibitor) and an analog of rolipram, which served as a prototype molecule for several development efforts.[3][4]: 668, 678 It was discontinued after a Phase II trial showed that its therapeutic window was too narrow; it could not be dosed high enough without causing significant side effects (nausea and vomiting), which was a problem with the rolipram class of molecules.[4]: 678
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
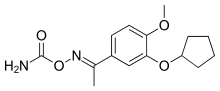
| Formula | C15H20N2O4 |
| Molar mass | 292.335 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
See also
References
- "Filaminist entry". Pubchem.
- Halpin DM (2008). "ABCD of the phosphodiesterase family: interaction and differential activity in COPD". International Journal of Chronic Obstructive Pulmonary Disease. 3 (4): 543–61. doi:10.2147/COPD.S1761. PMC 2650605. PMID 19281073.
- Giembycz MA (October 2008). "Can the anti-inflammatory potential of PDE4 inhibitors be realized: guarded optimism or wishful thinking?". British Journal of Pharmacology. 155 (3): 288–90. doi:10.1038/bjp.2008.297. PMC 2567889. PMID 18660832.
- McKenna JM, Muller GW (5 December 2006). "Medicinal Chemistry of PDE4 Inhibitors. Chapter 33". In Beavo JA, Francis SH, Houslay MD (eds.). Cyclic Nucleotide Phosphodiesterases in Health and Disease. CRC Press. ISBN 978-1-4200-2084-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.