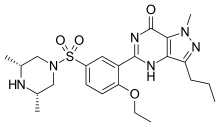
Aildenafil
Aildenafil (methisosildenafil) is a synthetic drug that is a structural analog of sildenafil (Viagra).[1] It was first reported in 2003,[2] and it is not approved by any health regulation agency. Like sildenafil, aildenafil is a phosphodiesterase type 5 inhibitor.
 | |
| Clinical data | |
|---|---|
| Other names | Methisosildenafil; Dimethyl sildenafil |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H32N6O4S |
| Molar mass | 488.61 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Aildenafil has been found as an adulterant in a variety of supplements which are sold as "natural" or "herbal" sexual enhancement products.[3][4][5][6][7] The United States Food and Drug Administration has warned consumers that any sexual enhancement product that claims to work as well as prescription products is likely to contain such a contaminant.[8]
References
- Zhao, Xia; Sun, Peihong; Zhou, Ying; Liu, Yuwang; Zhang, Huilin; Gu, Jingkai; Cui, Yimin (2009). "Pharmacokinetics and safety of aildenafil tablets in healthy Chinese male subjects after multiple dose administration". Zhongguo Linchuang Yaolixue Zazhi. 25 (2): 120–123.
- WO 2003016313, Liu B, "New compounds for treating impotence", published 2003-02-27
- Gryniewicz CM, Reepmeyer JC, Kauffman JF, Buhse LF (April 2009). "Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 49 (3): 601–606. doi:10.1016/j.jpba.2008.12.002. PMID 19150190.
- Choi DM, Park S, Yoon TH, Jeong HK, Pyo JS, Park J, et al. (2008). "Determination of analogs of sildenafil and vardenafil in foods by column liquid chromatography with a photodiode array detector, mass spectrometry, and nuclear magnetic resonance spectrometry". Journal of AOAC International. 91 (3): 580–588. doi:10.1093/jaoac/91.3.580. PMID 18567304.
- Reepmeyer JC, Woodruff JT (August 2007). "Use of liquid chromatography-mass spectrometry and a chemical cleavage reaction for the structure elucidation of a new sildenafil analogue detected as an adulterant in an herbal dietary supplement". Journal of Pharmaceutical and Biomedical Analysis. 44 (4): 887–893. doi:10.1016/j.jpba.2007.04.011. PMID 17532168.
- Reepmeyer JC, Woodruff JT, d'Avignon DA (April 2007). "Structure elucidation of a novel analogue of sildenafil detected as an adulterant in an herbal dietary supplement". Journal of Pharmaceutical and Biomedical Analysis. 43 (5): 1615–1621. doi:10.1016/j.jpba.2006.11.037. PMID 17207601.
- Enforcement Report for June 30, 2010, United States Food and Drug Administration
- Hidden Risks of Erectile Dysfunction "Treatments" Sold Online, United States Food and Drug Administration, February 21, 2009
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.