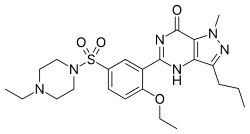
Homosildenafil
Homosildenafil (also known as methyl-sildenafil) is a synthetic drug which acts as a phosphodiesterase inhibitor.[1] It is an analog of sildenafil and vardenafil. Homosildenafil was first identified as an adulterant in sex enhancement products in 2003 and was more recently detected in dietary supplements.[2][3][4][5]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C23H32N6O4S |
| Molar mass | 488.61 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Homosildenafil has 35% the PDE5 inhibition activity of sildenafil itself with similar selectivity.[1]
Sildenafil is mainly metabolized by the microsomal isozymes CYP3A4 with secondary metabolism by CYP2C9. The major active metabolite is N-desmethylsildenafil. The plasma level of the equivalent homosildenafil metabolite reaches 40% of sildenafil's bioavailability. The N-desmethyl metabolite is further metabolized, with a half-life of 4 hours.[6][7][8][9]
See also
References
- Corbin JD, Beasley A, Blount MA, Francis SH (November 2004). "Vardenafil: structural basis for higher potency over sildenafil in inhibiting cGMP-specific phosphodiesterase-5 (PDE5)". Neurochemistry International. 45 (6): 859–63. doi:10.1016/j.neuint.2004.03.016. PMID 15312980. S2CID 25178860.
- Shin MH, Hong MK, Kim WS, Lee YJ, Jeoung YC (September 2003). "Identification of a new analogue of sildenafil added illegally to a functional food marketed for penile erectile dysfunction". Food Additives and Contaminants. 20 (9): 793–6. doi:10.1080/0265203031000121455. PMID 13129773. S2CID 30941705.
- Gryniewicz CM, Reepmeyer JC, Kauffman JF, Buhse LF (April 2009). "Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry". Journal of Pharmaceutical and Biomedical Analysis. 49 (3): 601–6. doi:10.1016/j.jpba.2008.12.002. PMID 19150190.
- "Advisory - "Forta for Men" sex enhancement product recalled; contains undeclared drug". PR Newswire. 2014. Retrieved 28 April 2015.
- Zou P, Oh SS, Hou P, Low MY, Koh HL (February 2006). "Simultaneous determination of synthetic phosphodiesterase-5 inhibitors found in a dietary supplement and pre-mixed bulk powders for dietary supplements using high-performance liquid chromatography with diode array detection and liquid chromatography-electrospray ionization tandem mass spectrometry". Journal of Chromatography A. 1104 (1–2): 113–22. doi:10.1016/j.chroma.2005.11.103. PMID 16364350.
- Tejada Inigo Saenz De; Kenneth M Ferguson; John S Whitaker (2002). "Daily treatment for erectile dysfunction using a pde5 inhibitor". Retrieved 27 April 2015.
- Ivan M. Borrello; Paolo Serafini; Kimberly A. Noonan; Vincenzo Bronte (2009). "Pde5 inhibitor compositions and methods for immunotherapy". Retrieved 27 April 2015.
- Ing-Jun Chen (2013). "Processes for preparing amine salts of sildenafil-analogues and use thereof". Retrieved 27 April 2015.
- 杨 许; 郭杰标; 黄志兵 (2009). "Preparation method for monoclonal antibody specifically binding vardenafil and analog thereof". Retrieved 27 April 2015.