Pazinaclone
Pazinaclone (DN-2327) is a sedative and anxiolytic drug in the cyclopyrrolone family of drugs. Some other cyclopyrrolone drugs include zopiclone and eszopiclone.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
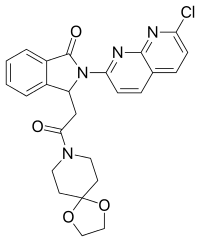
| Formula | C25H23ClN4O4 |
| Molar mass | 478.93 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Pazinaclone has a very similar pharmacological profile to the benzodiazepines including sedative and anxiolytic properties, but with less amnestic effects,[1] and at low doses it is a relatively selective anxiolytic, with sedative effects only appearing at higher doses.[2]
Pazinaclone produces its sedative and anxiolytic effects by acting as a partial agonist at GABAA benzodiazepine receptors, although pazinaclone is more subtype-selective than most benzodiazepines.[3]
Synthesis
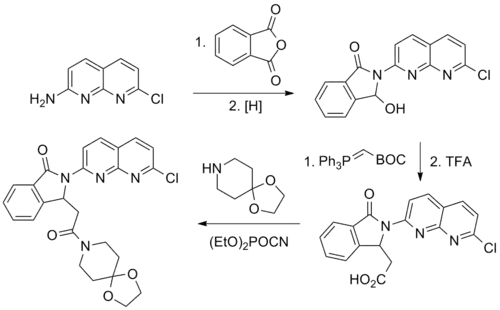
Reaction of 2-amino-7-chloro-1,8-naphthyridine with phthalic anhydride leads to the corresponding phthalimide. Selective reduction of one of the imide carbonyl groups in essence converts that to an aldehyde. Condensation with tert-butyl(triphenylphosphoranylidene)acetate gives the Wittig product.
The carboxylic acid is then treated with diethyl cyanophosphonate to convert that to an activated acid cyanide; reaction with 1,4-dioxa-8-azaspiro[4.5]decane results in formation of the corresponding amide, pazinaclone.
See also
References
- Wada T, Fukuda N (March 1992). "Effect of a new anxiolytic, DN-2327, on learning and memory in rats". Pharmacology, Biochemistry, and Behavior. 41 (3): 573–9. doi:10.1016/0091-3057(92)90375-p. PMID 1350101. S2CID 20581568.
- Suzuki M, Uchiumi M, Murasaki M (October 1995). "A comparative study of the psychological effects of DN-2327, a partial benzodiazepine agonist, and alprazolam". Psychopharmacology. 121 (4): 442–50. doi:10.1007/BF02246492. PMID 8619007. S2CID 35222663.
- Atack JR (May 2005). "The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics". Expert Opinion on Investigational Drugs. 14 (5): 601–18. doi:10.1517/13543784.14.5.601. PMID 15926867. S2CID 22793644.