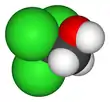
2,2,2-Trichloroethanol
2,2,2-Trichloroethanol is the chemical compound with formula Cl3C−CH2OH. Its molecule can be described as that of ethanol, with the three hydrogen atoms at position 2 (the methyl group) replaced by chlorine atoms. It is a clear flammable liquid at room temperature, colorless when pure but often with a light yellow color.[1][2]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2,2,2-Trichloroethan-1-ol | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
1697495 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.701 | ||
| EC Number |
| ||
Gmelin Reference |
2407 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
C2H3Cl3O | ||
| Molar mass | 149.40 g/mol | ||
| Density | 1.55 g/cm3 | ||
| Melting point | 17.8[1] °C (64.0 °F; 290.9 K) | ||
| Boiling point | 151[1] °C (304 °F; 424 K) | ||
| Hazards | |||
| GHS labelling: | |||
Pictograms |
  | ||
Signal word |
Danger | ||
Hazard statements |
H302, H315, H318, H336 | ||
Precautionary statements |
P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P310, P312, P321, P330, P332+P313, P362, P403+P233, P405, P501 | ||
| Flash point | 88 °C (190 °F; 361 K)[1] | ||
| Safety data sheet (SDS) | [1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
The pharmacological effects of this compound in humans are similar to those of its prodrug chloral hydrate, and of chlorobutanol. Historically, it has been used as a sedative hypnotic.[3] The hypnotic drug triclofos (2,2,2-trichloroethyl phosphate) is metabolized in vivo to 2,2,2-trichloroethanol. Chronic exposure may result in kidney and liver damage.[4]
2,2,2-Trichloroethanol can be added to SDS-PAGE gels in order to enable fluorescent detection of proteins without a staining step, for immunoblotting or other analysis methods.[5]
Use in organic synthesis
2,2,2-Trichloroethanol is an effective protecting group for carboxylic acids due to its ease in addition and removal.[6]
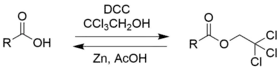
See also
- 1,1,1-Trichloroethane
- Tribromoethanol
- Triclofos
References
- "Material Safety Data Sheet- 2,2,2-Trichloroethanol, 98%" Online document at the Cole-Parmer website. Accessed on 2020-07-11.
- "2,2,2-Trichloroethanol ≥99%". Online product catalog page at Merck website. Accessed on 2020-07-11.
- The Merck Index, 13th Edition.
- S. Budavari; M. O'Neil; Ann Smith; P. Heckelman; J. Obenchain (15 March 1996). The Merck Index (12th print ed.). Taylor & Francis. ISBN 978-0-911910-12-4.
- Ladner, Carol (March 2004). "Visible fluorescent detection of proteins in polyacrylamide gels without staining". Analytical Biochemistry. 326 (1): 13–20. doi:10.1016/j.ab.2003.10.047. PMID 14769330.
- Lowder, Patrick D. (2001-04-15), "2,2,2-Trichloroethanol", Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rt203, ISBN 978-0471936237