Bilobalide
Bilobalide is a biologically active terpenic trilactone present in Ginkgo biloba.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.125.716 |
| Chemical and physical data | |
| Formula | C15H18O8 |
| Molar mass | 326.301 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Chemistry
Bilobalide is a main constituent of the terpenoids found in Ginkgo leaves. It also exists in minor amounts in the roots. It is a sesquiterpenoid, i.e. it has a 15-carbon skeleton. Its exact synthesis pathway from farnesyl pyrophosphate is still unknown.
Biosynthesis
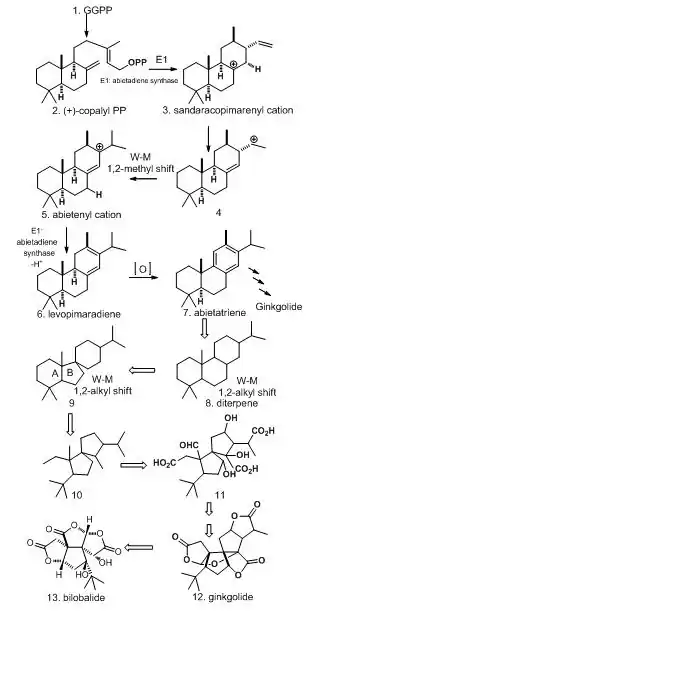
Bilobalide and ginkgolide have similar biosynthetic pathways. Bilobalide is formed by partially degraded ginkgolide. Bilobalide is derived from geranylgeranyl pyrophosphate (GGPP), which is formed by addition of farnesyl pyrophosphate (FPP) to an isopentenyl pyrophosphate (IPP) unit to form a C15 sesquiterpene. Such formation went through the mevalonate pathway (MVA) and methylerythritol phosphate MEP pathway. In order to generate bilobalide, C20 ginkgolide 13 must form first. To transform from GGPP to abietenyl cation 5, a single bifunctional enzyme abietadiene synthase E1 is required. However, due to the complexity of ginkgolide structures for rearrangement, ring cleavage, and formation of lactone rings, diterpene 8 is used to explain instead. Levopimaradiene 6 and abietatriene 7 are precursors for ginkgolide and bilobalide formation. The unusual tert-butyl substituent is formed from A ring cleavage in 9. Bilobalide 13 then formed in loss of carbons through degradation from ginkgolide 12, and lactones are formed from residual carboxyl and alcohol functions. The end product of bilobalide contains sesquiterpenes and three lactones units.[2]
Pharmacology
Bilobalide is important for producing several of the effects of Gingko biloba extracts, and it has neuroprotective effects,[3][4] as well as inducing the liver enzymes CYP3A1 and 1A2,[5] which may be partially responsible for interactions between gingko and other herbal medicines or pharmaceutical drugs. Bilobalide has recently been found to be a negative allosteric modulator at the GABAA and GABAA-rho receptors.[6][7] Of GABAA, it may possibly be selective for the subunits predominantly implicated in cognitive and memory functioning such as α1.
See also
- Ginkgolide
References
- van Beek TA, Montoro P (2009). "Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals". Journal of Chromatography A. 1216 (11): 2002–32. doi:10.1016/j.chroma.2009.01.013. PMID 19195661.
- Dewick, P. M. Medicinal Natural Products: Products:A Biosynthetic Approach. Third Edition ed.; Wiley&Sons: West Sussex, England, 2009; p 230-232.
- Defeudis FV (2002). "Bilobalide and neuroprotection". Pharmacological Research. 46 (6): 565–8. doi:10.1016/S1043-6618(02)00233-5. PMID 12457632.
- Kiewert C, Kumar V, Hildmann O, Hartmann J, Hillert M, Klein J (2008). "Role of glycine receptors and glycine release for the neuroprotective activity of bilobalide". Brain Research. 1201: 143–50. doi:10.1016/j.brainres.2008.01.052. PMID 18325484. S2CID 5191088.
- Deng Y, Bi HC, Zhao LZ, He F, Liu YQ, Yu JJ, Ou ZM, Ding L, Chen X, Huang ZY, Huang M, Zhou SF (2008). "Induction of cytochrome P450s by terpene trilactones and flavonoids of the Ginkgo biloba extract EGb 761 in rats". Xenobiotica. 38 (5): 465–81. doi:10.1080/00498250701883233. PMID 18421621. S2CID 84019088.
- http://sydney.edu.au/medicine/pharmacology/adrien-albert/images/pdfs/RefsPDFs/376.pdf
- "Archived copy" (PDF). sydney.edu.au. Archived from the original (PDF) on 9 November 2020. Retrieved 12 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
 Media related to Bilobalide at Wikimedia Commons
Media related to Bilobalide at Wikimedia Commons