Anandamide
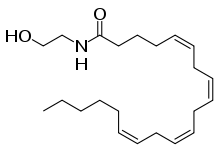
Anandamide (ANA), also known as N-arachidonoylethanolamine (AEA), is a fatty acid neurotransmitter. Anandamide was the first endocannabinoid to be discovered: it participates in the body's endocannabinoid system by binding to cannabinoid receptors, the same receptors that the psychoactive compound THC in cannabis acts on. Anandamide is found in nearly all tissues in a wide range of animals.[1] Anandamide has also been found in plants, including small amounts in chocolate.[2] The name 'anandamide' is taken from the Sanskrit word ananda, which means "joy, bliss, delight", plus amide.[3][4]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-tetraenamide | |
| Other names
N-arachidonoylethanolamine arachidonoylethanolamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Anandamide |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C22H37NO2 |
| Molar mass | 347.53 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Anandamide is derived from the non-oxidative metabolism of arachidonic acid, an essential omega-6 fatty acid. It is synthesized from N-arachidonoyl phosphatidylethanolamine by multiple pathways.[5] It is degraded primarily by the fatty acid amide hydrolase (FAAH) enzyme, which converts anandamide into ethanolamine and arachidonic acid. As such, inhibitors of FAAH lead to elevated anandamide levels and are being pursued for therapeutic use.[6][7]
Physiological functions
Anandamide was first described (and named) in 1992 by Raphael Mechoulam and his lab members W. A. Devane and Lumír Hanuš.[3]
Anandamide's effects can occur in either the central or peripheral nervous system. These distinct effects are mediated primarily by CB1 cannabinoid receptors in the central nervous system, and CB2 cannabinoid receptors in the periphery.[8] The latter are mainly involved in functions of the immune system. Cannabinoid receptors were originally discovered as being sensitive to Δ9-tetrahydrocannabinol (Δ9-THC, commonly called THC), which is the primary psychoactive cannabinoid found in cannabis. The discovery of anandamide came from research into CB1 and CB2, as it was inevitable that a naturally occurring (endogenous) chemical would be found to affect these receptors.
Anandamide is also important for implantation of the early stage embryo in its blastocyst form into the uterus. Therefore, cannabinoids such as Δ9-THC might influence processes during the earliest stages of human pregnancy.[9] Peak plasma anandamide occurs at ovulation and positively correlates with peak estradiol and gonadotrophin levels, suggesting that these may be involved in the regulation of anandamide levels.[10] Subsequently, anandamide has been proposed as a biomarker of infertility, but so far lacks any predictive values in order to be used clinically.[11]
The acute beneficial effects of exercise (termed as runner's high) seem to be mediated by anandamide in mice.[12] Anandamide is the precursor of a class of physiologically active substances, the prostamides.[13] Anandamide was found in 2007 to inhibit the proliferation of certain human breast cancer cell lines in vitro.[14]
Anandamide is found in chocolate together with two substances that might mimic the effects of anandamide, N-oleoylethanolamine and N-linoleoylethanolamine.[15]
Additionally, anandamide and other endocannabinoids are found in the model organism Drosophila melanogaster (fruit fly), although no CB receptors have been found in any insects.[16][17]
Effects on behavior
The role of the endocannabinoid system on behavior and mood is still being researched. Both the CB1 and CB2 receptors (the bonding site of anandamide) seem to play a role in the identification of positive and negative interpretation of environment and setting.[18] In animal models, anandamide plays a role in the interpretation of stimulus; specifically, optimism and pessimism in the presence of an ambiguous cue.[19] Anandamide has been shown to impair working memory in rats,[20] while THC (the compound in cannabis that binds to the CB1 and CB2 receptors) also shows a deficit in working memory.[21] Studies are under way to explore what role anandamide plays in human behavior, such as eating, sleep patterns, and pain relief.
This binding relationship of anandamide and the CB1/CB2 seems to play a role in neurotransmission of dopamine, serotonin, GABA, and glutamate.[22] There is currently encouraging, albeit embryonic, evidence for medicinal cannabis in the treatment of a range of psychiatric disorders. Supportive findings are emerging for some key isolates, however, clinicians need to be mindful of a range of prescriptive and occupational safety considerations, especially if initiating higher dose THC formulas..[23]
Anandamide injected directly into the forebrain reward-related brain structure nucleus accumbens enhances the pleasurable responses of rats to a rewarding sucrose taste, and enhances food intake as well.[24] Increasing anandamide seems to increase the intrinsic value of food, not necessarily by stimulation of appetite or hunger.[25]
Although more scientific information is needed, by comparing the effect of THC/anandamide, it can be inferred that anandamide plays a role in hunger, sleep, pain modulation, working memory, identification of novelty, and interpretation of environment.
Synthesis and degradation
In humans, anandamide is biosynthesized from N-arachidonoyl phosphatidylethanolamine (NAPE). In turn NAPE arises by transfer of arachidonic acid from lecithin to the free amine of cephalin through an N-acyltransferase enzyme.[26][27] Anandamide synthesis from NAPE occurs via multiple pathways and includes enzymes such as phospholipase A2, phospholipase C and N-acetylphosphatidylethanolamine-hydrolysing phospholipase D (NAPE-PLD).[5]
The crystal structure of NAPE-PLD in complex with phosphatidylethanolamine and deoxycholate shows how the cannabinoid anandamide is generated from membrane N-acylphosphatidylethanolamines (NAPEs), and reveals that bile acids – which are mainly involved in the absorption of lipids in the small intestine – modulate its biogenesis.[28]
Endogenous anandamide is present at very low levels and has a very short half-life due to the action of the enzyme fatty acid amide hydrolase (FAAH), which breaks it down into free arachidonic acid and ethanolamine. Studies of piglets show that dietary levels of arachidonic acid and other essential fatty acids affect the levels of anandamide and other endocannabinoids in the brain.[29] High fat diet feeding in mice increases levels of anandamide in the liver and increases lipogenesis.[30] This suggests that anandamide may play a role in the development of obesity, at least in rodents.
Paracetamol (called acetaminophen in the US and Canada) is metabolically combined with arachidonic acid by FAAH to form AM404.[31] This metabolite of paracetamol is a potent agonist at the TRPV1 vanilloid receptor, a weak agonist at both CB1 and CB2 receptors, and an inhibitor of anandamide reuptake. As a result, anandamide levels in the body and brain are elevated. In this fashion, paracetamol acts as a pro-drug for a cannabimimetic metabolite. This action may be partially or fully responsible for the analgesic effects of paracetamol.[32][33]
Endocannabinoid transporters for anandamide and 2-arachidonoylglycerol include the heat shock proteins (Hsp70s) and fatty acid binding proteins (FABPs).[34][35]
It is found that anandamide prefer cholesterol and ceramide more than other membrane lipids, and cholesterol can behave as a binding partner for it, and following an initial interaction mediated by the establishment of a hydrogen bond, the endocannabinoid is attracted towards the membrane interior, where it forms a molecular complex with cholesterol after a functional conformation adaptation to the apolar membrane milieu, and from there, the complex is further directed to the cannabinoid receptor (CB1) and out.[36]
Research and production
Black pepper contains the alkaloid guineesine, which is an anandamide reuptake inhibitor. It may therefore increase anandamide's physiological effects.[37]
Low dose intake of anandamide has an anxiolytic effect, but high dose intake injected directly into the cerebral fluid of the brain of mice shows evident cell apoptosis (programmed cell death) in vitro as opposed to necrosis.[38] Although this is contradicted by another study also done in both in vitro and in vivo showing neuron growth in the same situation.[39]
A Scottish woman with a rare genetic mutation in her FAAH gene with resultant elevated anandamide levels was reported to be immune to anxiety, unable to experience fear and insensitive to pain. The frequent burns and cuts she suffered due to her hypoalgesia healed more rapidly than was expected.[40][41][42]
See also
- Virodhamine
References
- Martin BR, Mechoulam R, Razdan RK (1999). "Discovery and characterization of endogenous cannabinoids". Life Sciences. 65 (6–7): 573–595. doi:10.1016/S0024-3205(99)00281-7. PMID 10462059.
- di Tomaso E, Beltramo M, Piomelli D (August 1996). "Brain cannabinoids in chocolate". Nature (Submitted manuscript). 382 (6593): 677–678. Bibcode:1996Natur.382..677D. doi:10.1038/382677a0. PMID 8751435. S2CID 4325706.
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. (December 1992). "Isolation and structure of a brain constituent that binds to the cannabinoid receptor". Science. 258 (5090): 1946–1949. Bibcode:1992Sci...258.1946D. doi:10.1126/science.1470919. PMID 1470919.
- Mechoulam R, Fride E (1995). "The unpaved road to the endogenous brain cannabinoid ligands, the anandamides". In Pertwee RG (ed.). Cannabinoid receptors. Boston: Academic Press. pp. 233–258. ISBN 978-0-12-551460-6.
- Wang J, Ueda N (September 2009). "Biology of endocannabinoid synthesis system". Prostaglandins & Other Lipid Mediators. 89 (3–4): 112–119. doi:10.1016/j.prostaglandins.2008.12.002. PMID 19126434.
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V (2009). "The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs". International Review of Neurobiology. 85: 57–72. doi:10.1016/S0074-7742(09)85005-8. ISBN 9780123748935. PMID 19607961.
- Hwang J, Adamson C, Butler D, Janero DR, Makriyannis A, Bahr BA (April 2010). "Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality". Life Sciences. 86 (15–16): 615–623. doi:10.1016/j.lfs.2009.06.003. PMC 2848893. PMID 19527737.
- Pacher P, Bátkai S, Kunos G (September 2006). "The endocannabinoid system as an emerging target of pharmacotherapy". Pharmacological Reviews. 58 (3): 389–462. doi:10.1124/pr.58.3.2. PMC 2241751. PMID 16968947.
- Piomelli D (January 2004). "THC: moderation during implantation". Nature Medicine. 10 (1): 19–20. doi:10.1038/nm0104-19. PMID 14702623. S2CID 29207064.
- El-Talatini MR, Taylor AH, Konje JC (April 2010). "The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle". Fertility and Sterility. 93 (6): 1989–1996. doi:10.1016/j.fertnstert.2008.12.033. PMID 19200965.
- Rapino C, Battista N, Bari M, Maccarrone M (2014). "Endocannabinoids as biomarkers of human reproduction". Human Reproduction Update. 20 (4): 501–516. doi:10.1093/humupd/dmu004. PMID 24516083.
- Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, Gass P (October 2015). "A runner's high depends on cannabinoid receptors in mice". Proceedings of the National Academy of Sciences of the United States of America. 112 (42): 13105–13108. Bibcode:2015PNAS..11213105F. doi:10.1073/pnas.1514996112. PMC 4620874. PMID 26438875.
- Woodward DF, Liang Y, Krauss AH (February 2008). "Prostamides (prostaglandin-ethanolamides) and their pharmacology". British Journal of Pharmacology. 153 (3): 410–419. doi:10.1038/sj.bjp.0707434. PMC 2241799. PMID 17721551.
- De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V (July 1998). "The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation". Proceedings of the National Academy of Sciences of the United States of America. 95 (14): 8375–8380. Bibcode:1998PNAS...95.8375D. doi:10.1073/pnas.95.14.8375. PMC 20983. PMID 9653194.
- di Tomaso E, Beltramo M, Piomelli D (August 1996). "Brain cannabinoids in chocolate". Nature. 382 (6593): 677–678. Bibcode:1996Natur.382..677D. doi:10.1038/382677a0. PMID 8751435. S2CID 4325706.
- Jeffries KA, Dempsey DR, Behari AL, Anderson RL, Merkler DJ (May 2014). "Drosophila melanogaster as a model system to study long-chain fatty acid amide metabolism". FEBS Letters. 588 (9): 1596–1602. doi:10.1016/j.febslet.2014.02.051. PMC 4023565. PMID 24650760.
- McPartland J, Di Marzo V, De Petrocellis L, Mercer A, Glass M (August 2001). "Cannabinoid receptors are absent in insects". The Journal of Comparative Neurology. 436 (4): 423–429. doi:10.1002/cne.1078. PMID 11447587. S2CID 25765136.
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R (June 2013). "Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences". Neuropsychology Review. 23 (2): 117–137. doi:10.1007/s11065-012-9222-1. PMC 3593817. PMID 23129391.
- Kregiel J, Malek N, Popik P, Starowicz K, Rygula R (February 2016). "Anandamide mediates cognitive judgement bias in rats". Neuropharmacology. 101: 146–153. doi:10.1016/j.neuropharm.2015.09.009. PMID 26363193.
- Mallet P, Beninger R (May 1996). "The endogenous cannabinoid receptor agonist anandamide impairs memory in rats". Behavioural Pharmacology. 7 (3): 276–284. doi:10.1097/00008877-199605000-00008. ISSN 0955-8810.
- Calabrese EJ, Rubio-Casillas A (May 2018). "Biphasic effects of THC in memory and cognition". European Journal of Clinical Investigation. 48 (5): e12920. doi:10.1111/eci.12920. PMID 29574698.
- Fantegrossi WE, Wilson CD, Berquist MD (February 2018). "Pro-psychotic effects of synthetic cannabinoids: interactions with central dopamine, serotonin, and glutamate systems". Drug Metabolism Reviews. 50 (1): 65–73. doi:10.1080/03602532.2018.1428343. PMC 6419500. PMID 29385930.
- Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J (January 2020). "Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review". BMC Psychiatry. 20 (1): 24. doi:10.1186/s12888-019-2409-8. PMC 6966847. PMID 31948424.
- Mahler SV, Smith KS, Berridge KC (November 2007). "Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward". Neuropsychopharmacology. 32 (11): 2267–2278. doi:10.1038/sj.npp.1301376. PMID 17406653.
- Williams CM, Kirkham TC (June 2002). "Observational analysis of feeding induced by Δ9-THC and anandamide". Physiology & Behavior. 76 (2): 241–250. doi:10.1016/S0031-9384(02)00725-4. PMID 12044596.
- Natarajan V, Reddy PV, Schmid PC, Schmid HH (August 1982). "N-Acylation of ethanolamine phospholipids in canine myocardium". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 712 (2): 342–355. doi:10.1016/0005-2760(82)90352-6. PMID 7126608.
- Cadas H, di Tomaso E, Piomelli D (February 1997). "Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain". The Journal of Neuroscience. 17 (4): 1226–1242. doi:10.1523/JNEUROSCI.17-04-01226.1997. PMC 6793739. PMID 9006968.
- Magotti P, Bauer I, Igarashi M, Babagoli M, Marotta R, Piomelli D, Garau G (March 2015). "Structure of human N-acylphosphatidylethanolamine-hydrolyzing phospholipase D: regulation of fatty acid ethanolamide biosynthesis by bile acids". Structure. 23 (3): 598–604. doi:10.1016/j.str.2014.12.018. PMC 4351732. PMID 25684574.
- Berger A, Crozier G, Bisogno T, Cavaliere P, Innis S, Di Marzo V (May 2001). "Anandamide and diet: inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets". Proceedings of the National Academy of Sciences of the United States of America. 98 (11): 6402–6406. Bibcode:2001PNAS...98.6402B. doi:10.1073/pnas.101119098. PMC 33480. PMID 11353819.
- Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Bátkai S, et al. (May 2005). "Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity". The Journal of Clinical Investigation. 115 (5): 1298–1305. doi:10.1172/JCI23057. PMC 1087161. PMID 15864349.
- Högestätt ED, Jönsson BA, Ermund A, Andersson DA, Björk H, Alexander JP, et al. (September 2005). "Conversion of acetaminophen to the bioactive N-acylphenolamine AM404 via fatty acid amide hydrolase-dependent arachidonic acid conjugation in the nervous system". The Journal of Biological Chemistry. 280 (36): 31405–31412. doi:10.1074/jbc.M501489200. PMID 15987694.
- Bertolini A, Ferrari A, Ottani A, Guerzoni S, Tacchi R, Leone S (September 2006). "Paracetamol: new vistas of an old drug". CNS Drug Reviews. 12 (3–4): 250–275. doi:10.1111/j.1527-3458.2006.00250.x. PMC 6506194. PMID 17227290.
- Sinning C, Watzer B, Coste O, Nüsing RM, Ott I, Ligresti A, et al. (December 2008). "New analgesics synthetically derived from the paracetamol metabolite N-(4-hydroxyphenyl)-(5Z,8Z,11Z,14Z)-icosatetra-5,8,11,14-enamide". Journal of Medicinal Chemistry. 51 (24): 7800–7805. doi:10.1021/jm800807k. PMID 19053765.
- Kaczocha M, Glaser ST, Deutsch DG (April 2009). "Identification of intracellular carriers for the endocannabinoid anandamide". Proceedings of the National Academy of Sciences of the United States of America. 106 (15): 6375–6380. Bibcode:2009PNAS..106.6375K. doi:10.1073/pnas.0901515106. PMC 2669397. PMID 19307565.
- Oddi S, Fezza F, Pasquariello N, D'Agostino A, Catanzaro G, De Simone C, et al. (June 2009). "Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins". Chemistry & Biology. 16 (6): 624–632. doi:10.1016/j.chembiol.2009.05.004. PMID 19481477.
- Di Scala C, Fantini J, Yahi N, Barrantes FJ, Chahinian H (May 2018). "Anandamide Revisited: How Cholesterol and Ceramides Control Receptor-Dependent and Receptor-Independent Signal Transmission Pathways of a Lipid Neurotransmitter". Biomolecules. 8 (2): 31. doi:10.3390/biom8020031. PMC 6022874. PMID 29789479.
- Nicolussi S, Viveros-Paredes JM, Gachet MS, Rau M, Flores-Soto ME, Blunder M, Gertsch J (February 2014). "Guineensine is a novel inhibitor of endocannabinoid uptake showing cannabimimetic behavioral effects in BALB/c mice". Pharmacological Research. 80: 52–65. doi:10.1016/j.phrs.2013.12.010. PMID 24412246.
- Cernak I, Vink R, Natale J, Stoica B, Lea PM, Movsesyan V, et al. (May 2004). "The "dark side" of endocannabinoids: a neurotoxic role for anandamide". Journal of Cerebral Blood Flow and Metabolism. 24 (5): 564–578. doi:10.1097/00004647-200405000-00011. PMID 15129189.
- Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, et al. (May 2003). "Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases". The Journal of Neuroscience. 23 (10): 4127–4133. doi:10.1523/JNEUROSCI.23-10-04127.2003. PMC 6741091. PMID 12764100.
- Habib AM, Okorokov AL, Hill MN, Bras JT, Lee MC, Li S, et al. (August 2019). "Microdeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivity". British Journal of Anaesthesia. 123 (2): e249–e253. doi:10.1016/j.bja.2019.02.019. PMC 6676009. PMID 30929760.
- Murphy H (28 March 2019). "At 71, She's Never Felt Pain or Anxiety. Now Scientists Know Why". The New York Times. Retrieved 29 March 2019.
- Sample I (28 March 2019). "Scientists find genetic mutation that makes woman feel no pain". The Guardian. Retrieved 29 March 2019.
External links
- Could anandamide be the missing link to "runner's high"? Accessed 2015-10-31
- Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A (December 2003). "Exercise activates the endocannabinoid system". NeuroReport. 14 (17): 2209–2211. doi:10.1097/00001756-200312020-00015. PMID 14625449. S2CID 1971671.
