Tetrahydrocannabutol
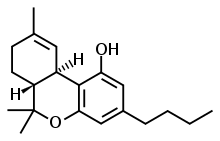
Δ9-Tetrahydrocannabutol (tetrahydrocannabinol-C4, THC-C4, Δ9-THCB, (C4)-Δ9-THC, butyl-THC) is a homologue of tetrahydrocannabinol (THC), the active component of cannabis.[1] They are only different by the pentyl side chain being replaced by a butyl side chain. Δ9-THCB, showed an affinity for the human CB1 (Ki = 15 nM) and CB2 receptors (Ki = 51 nM) comparable to that of Δ9-THC.[1] The formalin test in vivo was performed on Δ9-THCB in order to reveal possible analgesic and anti-inflammatory properties.[1] The tetrad test in mice showed a partial agonistic activity of Δ9-THCB toward the CB1 receptor.[1] The propyl analog, THCV, is a cannabinoid receptor type 1 and cannabinoid receptor type 2 antagonist,[2] while THC is a CB1 agonist. THCB has rarely been isolated from cannabis samples,[1][3] but appears to be less commonly present than THC or THCV. It is metabolised in a similar manner to THC.[4] Similarly to THC, it has 7 double bond isomers and 30 stereoisomers.[5] The Δ8 isomer is known as a synthetic cannabinoid under the code name JWH-130,[6] and the ring-opened analogue cannibidibutol is also known.[7]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H28O2 |
| Molar mass | 300.442 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Legality
THCB is not scheduled internationally under the Convention on Psychotropic Substances, but may be controlled under analogue law in some individual jurisdictions as a homologue of THC.
See also
- Cannabis
- Cannabinoid
- Parahexyl
- Perrottetinene
- Tetrahydrocannabihexol
- Tetrahydrocannabiphorol
References
- Linciano, Pasquale; Citti, Cinzia; Luongo, Livio; Belardo, Carmela; Maione, Sabatino; Vandelli, Maria Angela; Forni, Flavio; Gigli, Giuseppe; Laganà, Aldo; Montone, Carmela Maria; Cannazza, Giuseppe (2020-01-24). "Isolation of a High-Affinity Cannabinoid for the Human CB1 Receptor from a Medicinal Cannabis sativa Variety: Δ 9 -Tetrahydrocannabutol, the Butyl Homologue of Δ 9 -Tetrahydrocannabinol". Journal of Natural Products. 83 (1): 88–98. doi:10.1021/acs.jnatprod.9b00876. ISSN 0163-3864. PMID 31891265. S2CID 209519659.
- Thomas A, Stevenson LA, Wease KN, Price MR, Baillie G, Ross RA, Pertwee RG (December 2005). "Evidence that the plant cannabinoid Delta9-tetrahydrocannabivarin is a cannabinoid CB1 and CB2 receptor antagonist". British Journal of Pharmacology. 146 (7): 917–26. doi:10.1038/sj.bjp.0706414. PMC 1751228. PMID 16205722.
- Harvey DJ (April 1976). "Characterization of the butyl homologues of delta1-tetrahydrocannabinol, cannabinol and cannabidiol in samples of cannabis by combined gas chromatography and mass spectrometry". The Journal of Pharmacy and Pharmacology. 28 (4): 280–5. doi:10.1111/j.2042-7158.1976.tb04153.x. PMID 6715. S2CID 32734030.
- Brown NK, Harvey DJ (April 1988). "In vivo metabolism of the n-butyl-homologues of delta 9-tetrahydrocannabinol and delta 8-tetrahydrocannabinol by the mouse". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 18 (4): 417–27. doi:10.3109/00498258809041678. PMID 2840781.
- "Verschil THC Olie, CBD olie, wietolie, hennepolie en cannabisolie?". Dutch-Headshop.com. Retrieved 19 November 2016.
- Bow EW, Rimoldi JM (2016). "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry. 8: 17–39. doi:10.4137/PMC.S32171. PMC 4927043. PMID 27398024.
- Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G (November 2016). "Phytocannabinoids: a unified critical inventory". Natural Product Reports. 33 (12): 1357–1392. doi:10.1039/c6np00074f. PMID 27722705.

