AMB-FUBINACA
AMB-FUBINACA (also known as FUB-AMB and MMB-FUBINACA) is an indazole-based synthetic cannabinoid that is a potent agonist for the cannabinoid receptors, with Ki values of 10.04 nM at CB1 and 0.786 nM at CB2 and EC50 values of 0.5433 nM at CB1 and 0.1278 nM at CB2,[1] and has been sold online as a designer drug.[2][3][4][5] It was originally developed by Pfizer which described the compound in a patent in 2009, but was later abandoned and never tested on humans.[6] AMB-FUBINACA was the most common synthetic cannabinoid identified in drug seizures by the Drug Enforcement Administration in 2017 and the first half of 2018.[7]
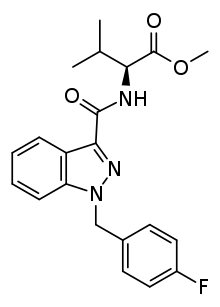 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H22FN3O3 |
| Molar mass | 383.423 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mass casualties
On July 12, 2016, the New York City Emergency Medical Services responded[8] to a "mass casualty event" in Brooklyn, New York,[6] where 33 people ranging in age from 25 to 59 years old were adversely affected by the drug.[8] 18 were hospitalized.[8] All of the victims were described by-standers as “zombielike” and the cause was attributed to use of AMB-FUBINACA as the demethylated metabolite was found in the blood and urine of eight of the hospitalized patients that had been sent for testing by the DEA. Screening for the more usual drugs of abuse was negative in all 8 patients. AMB-FUBINACA itself was found in a sample from the product smoked by another patient. The metabolite was identified after 10 days and the AMB-FUBINACA was only confirmed 17 days after the incident.[8]
Around 60 deaths in New Zealand were attributed to either AMB-FUBINACA or a related compound 5F-ADB during 2017–2018, with tested products containing between 32 mg/g and 400 mg/g of the active ingredient, between 2x to 25x stronger than the product involved in the mass casualty event in New York a year earlier.[9][10][11][12]
Legal status
In the United States, AMB-FUBINACA is a Schedule I Controlled Substance.[13] Prior to being scheduled at the federal level, the state of Louisiana banned AMB-FUBINACA through an emergency rule after it was detected in a synthetic cannabis product called "Train Wreck 2" which had been linked to adverse events and seizures on 3 June 2014.[14]
Sweden's public health agency suggested classifying AMB-FUBINACA as a hazardous substance on November 10, 2014.[15]
See also
References
- Gamage TF, Farquhar CE, Lefever TW, Marusich JA, Kevin RC, McGregor IS, et al. (May 2018). "Molecular and Behavioral Pharmacological Characterization of Abused Synthetic Cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA". The Journal of Pharmacology and Experimental Therapeutics. 365 (2): 437–446. doi:10.1124/jpet.117.246983. PMC 5932312. PMID 29549157.
- "FUB-AMB". Cayman Chemical. Retrieved 21 July 2015.
- Akamatsu S, Yoshida M (January 2016). "Fragmentation of synthetic cannabinoids with an isopropyl group or a tert-butyl group ionized by electron impact and electrospray". Journal of Mass Spectrometry. 51 (1): 28–32. Bibcode:2016JMSp...51...28A. doi:10.1002/jms.3722. PMID 26757069.
- Banister SD, Longworth M, Kevin R, Sachdev S, Santiago M, Stuart J, et al. (September 2016). "Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues". ACS Chemical Neuroscience. 7 (9): 1241–54. doi:10.1021/acschemneuro.6b00137. PMID 27421060.
- Wagmann, Lea; Stiller, Rebecca G.; Fischmann, Svenja; Westphal, Folker; Meyer, Markus R. (July 2022). "Going deeper into the toxicokinetics of synthetic cannabinoids: in vitro contribution of human carboxylesterases". Archives of Toxicology. 96 (10): 2755–2766. doi:10.1007/s00204-022-03332-z. ISSN 1432-0738. PMC 9352624. PMID 35788413.
- Santora M (2016-12-14). "Drug 85 Times as Potent as Marijuana Caused a 'Zombielike' State in Brooklyn". The New York Times. ISSN 0362-4331. Retrieved 2016-12-15.
- Yin S (2019). "Adolescents and Drug Abuse: 21st Century Synthetic Substances". Clinical Pediatric Emergency Medicine. 20 (1): 17–24. doi:10.1016/j.cpem.2019.03.003. S2CID 88290992.
- Adams AJ, Banister SD, Irizarry L, Trecki J, Schwartz M, Gerona R (January 2017). ""Zombie" Outbreak Caused by the Synthetic Cannabinoid AMB-FUBINACA in New York" (PDF). The New England Journal of Medicine. 376 (3): 235–242. doi:10.1056/NEJMoa1610300. PMID 27973993. S2CID 205100837.
- Killer chemicals, Part 1. Stuff.co.nz, September 2017
- Worsening synthetic drug crisis demands action. Stuff.co.nz, 18 September 2017
- "Synthetic cannabis users gambling with their lives after a 'bad batch'". stuff.co.nz.
- "Synthetic cannabis a 'public health emergency' - NZ Drug Foundation | Newshub". Archived from the original on 2018-10-18. Retrieved 2018-10-17.
- Controlled Substances listed by the DEA
- "DHH Adds Two New Synthetic Marijuana Compounds to Banned List". Louisiana Department of Health and Hospitals. 3 June 2014. Retrieved 21 July 2015.
- "Cannabinoider föreslås bli klassade som hälsofarlig vara" (in Swedish). Folkhälsomyndigheten. Retrieved 21 July 2015.