THC morpholinylbutyrate
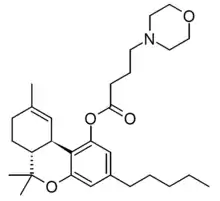
THC morpholinylbutyrate (SP-111, Δ9-THC-O-[4-(morpholin-4-yl)butyrate]) is a synthetic derivative of tetrahydrocannabinol, developed in the 1970s. It is a prodrug which is converted into THC inside the body, and was one of the first derivatives of THC that is able to form water-soluble salts, giving it a significant advantage over THC for some applications. However, it is less potent than THC and the metabolic conversion to THC is relatively slow and variable, giving it unpredictable pharmacokinetics which has limited its research applications.[1][2][3][4]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C29H43NO4 |
| Molar mass | 469.666 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- Dykstra LA, McMillan DE, Harris LS. "Effects of delta-9-THC and a water soluble ester of delta-9-THC on schedule-controlled behavior". Pharmacology, Biochemistry, and Behavior. 3 (1): 29–32. doi:10.1016/0091-3057(75)90077-5. PMID 1129353.
- Segal M (January 1978). "The effects of SP-111, a water-soluble THC derivative, on neuronal activity in the rat brain". Brain Research. 139 (2): 263–75. doi:10.1016/0006-8993(78)90928-9. PMID 624059.
- Hershkowitz M, Szechtman H (November 1979). "Pretreatment with delta 1-tetrahydrocannabinol and psychoactive drugs: effects on uptake of biogenic amines and on behavior". European Journal of Pharmacology. 59 (3–4): 267–76. doi:10.1016/0014-2999(79)90290-5. PMID 230975.
- Järbe TU, McMillan DE. "delta 9-THC as a discriminative stimulus in rats and pigeons: generalization to THC metabolites and SP-111". Psychopharmacology. 71 (3): 281–9. doi:10.1007/BF00433063. PMID 6256796.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.