THC hemisuccinate
THC hemisuccinate (Δ9-THC-O-hemisuccinate, Dronabinol hemisuccinate) is a synthetic derivative of tetrahydrocannabinol, developed in the 1990s. It is a water-soluble prodrug ester which is converted into THC inside the body, and was developed to overcome the poor bioavailability of THC when taken by non-inhaled routes of administration.[1][2][3]
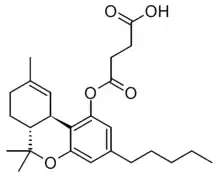 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H34O5 |
| Molar mass | 414.542 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- Elsohly MA, Little TL, Hikal A, Harland E, Stanford DF, Walker L (November 1991). "Rectal bioavailability of delta-9-tetrahydrocannabinol from various esters". Pharmacology, Biochemistry, and Behavior. 40 (3): 497–502. doi:10.1016/0091-3057(91)90353-4. PMID 1666913.
- ElSohly MA, Stanford DF, Harland EC, Hikal AH, Walker LA, Little TL, et al. (October 1991). "Rectal bioavailability of delta-9-tetrahydrocannabinol from the hemisuccinate ester in monkeys". Journal of Pharmaceutical Sciences. 80 (10): 942–5. doi:10.1002/jps.2600801008. PMID 1664466.
- Upadhye SB, Kulkarni SJ, Majumdar S, Avery MA, Gul W, ElSohly MA, Repka MA (June 2010). "Preparation and characterization of inclusion complexes of a hemisuccinate ester prodrug of delta9-tetrahydrocannabinol with modified beta-cyclodextrins". AAPS PharmSciTech. 11 (2): 509–17. doi:10.1208/s12249-010-9401-4. PMC 2902337. PMID 20333489.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.