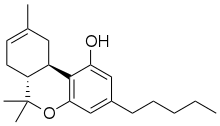
delta-8-Tetrahydrocannabinol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
6,6,9-trimethyl-3-pentyl-6a,7,10,10a-tetrahydrobenzo[c]chromen-1-ol | |
Other names
| |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.165.076 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C21H30O2 |
| Molar mass | 314.5 g/mol |
| Density | 1.0±0.1 g/cm3 |
| Boiling point | 383.5±42.0 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Delta-8-tetrahydrocannabinol (delta-8-THC, Δ8-THC) is a psychoactive cannabinoid found in the Cannabis plant.[1][2][3] It is an isomer of delta-9-tetrahydrocannabinol (delta-9-THC, Δ9-THC), the compound commonly known as THC. ∆8-THC is under preliminary research for its biological properties.[4] Delta-8-THC has not been approved by the FDA thus far for public use and should not be used around children or pets. [5]
Effects
∆8-THC is moderately less potent than Δ9-THC (being ≈50-66% as potent in-vivo).[6][7] This essentially means that it has properties similar to those of ∆9-THC, although to a lesser degree per milligram of material consumed.[8] Delta-8-THC and delta-9-THC both contain a double bond in their molecular chain, but the location is different. Delta-8-THC has the bond in the 8th carbon chain while delta-9 contains it in the 9th carbon chain.
Although delta-8-THC functions similarly to delta-9 in many ways, it appears to be only two-thirds as psychoactive. This may be because it bonds differently to CB1, the cannabinoid receptor that regulates much of THC’s mind-altering effect.[9] ∆8-THC may cause increased heart rate, reddening of the eyes, dizziness, dryness of the mouth and throat, paresthesia, tinnitus, increased body awareness, weakness, muscle tension or tremor, reduced motor coordination, fatigue, sleepiness, changes in visual perception, altered visual imagery, enhancement of colors or contrasts, time distortion, changes in auditory perception, euphoria, tranquility, relaxation, racing thoughts, dreamy introspective states, or difficulty in thinking, speaking, reading, or remembering.[7]
A 1973 study testing the effects of ∆8-THC in dogs and monkeys reported that a single oral dose of 9,000 milligrams per kilogram of body mass (mg/kg) was nonlethal in all dogs and monkeys studied.[10][11] The same study reported that the median lethal dose of ∆8-THC in rats was comparable to that of ∆9-THC.[10] Both isomers of THC have been found to cause a transient increase in blood pressure in rats,[12] though the effects of cannabinoids on the cardiovascular system are complex.[13] Animal studies indicate that ∆8-THC exerts many of its central effects by binding to cannabinoid receptors found in various regions of the brain, including the cerebral cortex, thalamus, basal ganglia, hippocampus, and cerebellum.[14][15]
Pharmacology
Pharmacodynamics
The pharmacodynamic profile of ∆8-THC is similar to that of ∆9-THC.[6][7] It is a partial agonist of CB1 and CB2 cannabinoid receptors with about half the potency of ∆9-THC in most but not all measures of biological activity.[16][17][18] ∆8-THC has been reported to have a Ki value of 44 ± 12 nM at the CB1 receptor and 44 ± 17 nM at the CB2 receptor.[19] These values are higher than those typically reported for ∆9-THC (CB1 Ki = 40.7 nM) at the same receptors, indicating that ∆8-THC binds to cannabinoid receptors less efficiently than ∆9-THC.[20]
Pharmacokinetics
The pharmacokinetic profile of ∆8-THC is also similar to that of ∆9-THC.[6][7] Following ingestion in humans, hepatic cytochrome P450 enzymes including CYP2C9 and CYP3A4 first convert ∆8-THC into 11-hydroxy-Δ8-tetrahydrocannabinol (11-OH-Δ8-THC).[21][22] Next, dehydrogenase enzymes convert 11-OH-Δ8-THC into 11-nor-Δ8-tetrahydrocannabinol-9-carboxylic acid (11-nor-Δ8-THC-9-COOH, also known as Δ8-THC-11-oic acid).[22][23] Finally, Δ8-THC-11-oic acid undergoes glucuronidation by glucuronidase enzymes to form 11-nor-Δ8-tetrahydrocannabinol-9-carboxylic acid glucuronide (Δ8-THC-COOH-glu).[22][23] This final product is then excreted in the urine.[24][25]
Physical and chemical properties
∆8-THC is a tricyclic terpenoid. Although it has the same chemical formula as ∆9-THC, one of its carbon-carbon double bonds is located in a different position.[6] This difference in structure increases the chemical stability of ∆8-THC relative to ∆9-THC, lengthening shelf life and allowing the compound to resist undergoing oxidation to cannabinol over time.[16] Like other cannabinoids, ∆8-THC is very lipophilic (log P = 7.4[26]). It is an extremely viscous, colorless oil at room temperature.[27]
While ∆8-THC is naturally found in plants of the Cannabis genus,[3] this compound can also be produced in an industrial or laboratory setting by exposing CBD to acids and heat.[28][29][30] Solvents that may be used during this process include methylene chloride, toluene, and hexane.[30] Acids that may be used include tosylic acid, indium(III) triflate, trimethylsilyl trifluoromethanesulfonate, hydrochloric acid, and sulfuric acid.[30][31] Because it is possible for chemical contaminants to be generated during the process of converting CBD to ∆8-THC, such as Δ10-THC, 9-OH-HHC and other side products, as well as the potentially toxic chemical reagents used during manufacture, concern has been raised about the safety of untested or impure ∆8-THC products.[31][32]
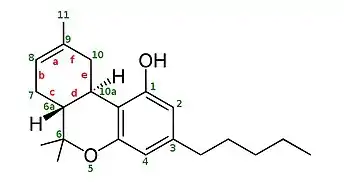

∆8-THC has a double bond (a) between the carbon atoms labeled 8 and 9. ∆9-THC has a double bond (a) between the carbon atoms labeled 9 and 10.
The ongoing controversy regarding the legal status of ∆8-THC in the U.S. is complicated by chemical nomenclature. According to a 2019 literature review published in Clinical Toxicology, the term "synthetic cannabinoid" typically refers to a full agonist of CB1 and CB2 cannabinoid receptors.[33] According to the review, "The psychoactive (and probably the toxic) effects of synthetic cannabinoid receptor agonists are likely due to their action as full receptor agonists and their greater potency at CB1 receptors."[33] Because ∆8-THC and ∆9-THC are partial agonists of cannabinoid receptors,[17] rather than full agonists, these compounds are less potent and less toxic than many synthetic cannabinoids.[34] Although it has not been definitively proven if full agonism is the reason for toxicity as Delta-9-THC has been shown to act as a full CB1 agonist on specific CB1 receptors located in the hippocampus section of the brain.[35] and the synthetic cannabinoid EG-018 acts as a partial agonist[36] The classical cannabinoid dibenzopyran structure class of drugs which includes THC interact with a different spot inside of the CB1 receptor than synthetic cannabinoid compounds of unrelated chemical classes such as Naphthoylindoles do which may contribute to toxicity.[37]
History
The partial synthesis of ∆8-THC was published in 1941 by Roger Adams and colleagues at the University of Illinois.[38] In 1942, the same research group studied its physiological and psychoactive effects after oral dosing in human volunteers.[39] Total syntheses of ∆8-THC were achieved by 1965.[40] In 1966, the chemical structure of ∆8-THC isolated from cannabis was characterized using modern methods by Richard L. Hively, William A. Mosher, and Friedrich W. Hoffmann at the University of Delaware.[41] A stereospecific synthesis of ∆8-THC from olivetol and verbenol was reported by Raphael Mechoulam and colleagues at the Weizmann Institute of Science in 1967.[42] ∆8-THC was often referred to as "Delta-6-THC" (Δ6-THC) in early scientific literature, but this name is no longer conventional among most authors.[43]
Legality in the United States
In 1937, Delta-9 THC was made illegal with the passage of the Marihuana Tax Act, which made cannabis illegal on a federal level. Over the course of the 1970s, 11 states decriminalized marijuana, with others reducing related penalties. In 1976, a parent’s movement started which influenced public attitudes, leading to the war on drugs in the 1980s. President Ronald Reagan re-enacted mandatory sentences for cannabis-related offenses.[44]
The 2018 United States farm bill signed into law in December 2018 states "that any cannabis containing under 0.3% Delta-9 THC is classified as “hemp” and no longer a controlled substance.",[45] ∆8-THC products partially synthesized from compliant sources (including industrial hemp and derivative cannabidiol extracts) have been sold by a range of digital vendors and a more limited array of brick and mortar retailers, including head shops. Ranging from bulk quantities of unrefined distillate to prepared edibles and atomizer cartridges[46] suffused with cannabis-derived terpenes, they are usually marketed as federally legal alternatives to their ∆9-THC counterparts.[47] However, the legal status of ∆8-THC at the federal level is in question with some believing that the Oct. 2020 DEA IFR addressing "synthetics" applied to Delta-8 and other hemp derivatives allowed by the Farm Bill.[48][49]
Beginning in late 2020, ∆8-THC began to attract the attention of many cannabis consumers throughout the United States. Thought of as an alternative to traditional cannabis use, especially in areas where marijuana is illegal, the news of ∆8-THC spread quickly via news agencies,[49] cannabis publications,[47] blogs, and podcasts which attracted a storm of social media attention.
Because marketing of ∆8-THC products does not require special licensing or, in most states, chemical analysis, the fact that these products can be produced and marketed nationally instead of one state at a time, and have no more tax than an ordinary sales tax, if that, means that they are far cheaper than ∆9-THC products purchased in a cannabis dispensary. They can be sent through the mail; ∆9-THC products, still illegal at the federal level, can not. As of 2021, a sizeable new industry, including franchises, is being or has already been created.
As of early 2021, "Delta-8"/∆8-THC is one of the fastest growing segments of products derived from hemp.[50]
Despite claims of legality by manufacturers, independent testing of products from retail often reveals significant levels of delta 9 THC, well above the legal threshold.[51] One store owner in Menomonee Falls, Wisconsin is facing a sentence of up to 50 years for selling delta 8 products with illegal amounts of delta 9 THC.[52] Other raids and arrests have happened due to delta 9 THC content of these products in North Carolina.[53][54] Catoosa County Sheriff Sisk has announced intent to prosecute stores distributing delta 8 THC with non-compliant delta 9 THC levels, and has stated “the products the sheriffs office has purchased and tested all contain significant levels of Delta 9.” and that they have “evidence needed to move forward with prosecution and seizures."[55]
Delta-8-THC products have been sold in regulated recreational Cannabis and medical Cannabis industries within the United States for over 2 years. Including California's regulated recreational Cannabis industry [56] and Pennsylvania's regulated medical Cannabis industry[57][58] both with products containing Delta-8-THC at levels unnaturally higher than what the plant can produce suggesting chemical conversion.
The states of Michigan and Oregon have regulated Delta-8-THC products to be sold under their respective regulated Cannabis systems.[59][60]
U.S. Court of Appeals for the Ninth Circuit panel decided on May 20, 2022, by a vote of 3 to 0, that the 2018 Farm Bill does not classify delta-8 goods as marijuana. [61]
Safety concerns
∆8-THC is typically synthesized from cannabidiol extracted from hemp[62] as the natural quantities of Delta 8 found in hemp are low. The reaction often yields a mixture that contains other cannabinoids and unknown reaction by-products. As a result, most products sold as ∆8-THC are not actually pure ∆8-THC.[62] Little is known about the identity and the health effects of the impurities.[62]
The FDA has reported 104 adverse event reports related to delta 8 THC, including one pediatric case with a coded outcome of "death".[63] National poison control centers received 2,362 exposure cases of delta-8 THC products between January 1, 2021 (i.e., date that delta-8 THC product code was added to database), and February 28, 2022, 58% of these exposures involve adults.[63]
Research
∆8-THC has been studied as a potential treatment for glaucoma,[64][65] corneal injury,[66][67] and chemotherapy-induced nausea and vomiting.[16]
Although ∆8-THC is a minor constituent of medical cannabis, no large clinical studies have been conducted on it alone. However, thus far the pharmacology of the compound has been found to be similar to its isomer delta-9-THC, though with less potency.
See also
- Cannabinoid
- Cannabis (drug)
- 7,8-Dihydrocannabinol
- 11-Hydroxy-Delta-8-THC
- delta-3-Tetrahydrocannabinol
- delta-4-Tetrahydrocannabinol
- delta-7-Tetrahydrocannabinol (delta-5-tetrahydrocannabinol)
- delta-10-Tetrahydrocannabinol (delta-2-tetrahydrocannabinol)
- delta-6-Cannabidiol
- Hexahydrocannabinol
- Tetrahydrocannabinol
- Tetrahydrocannabutol
- Tetrahydrocannabiphorol
- THC-O-acetate
- THC-O-phosphate
- Ajulemic acid
- Endocannabinoid system
References
- "NCI Drug Dictionary". National Cancer Institute. 2 February 2011. Retrieved 10 November 2020.
- "Δ8-Tetrahydrocannabinol". webbook.nist.gov. U.S. Secretary of Commerce. Retrieved 10 November 2020.
- Qamar S, Manrique YJ, Parekh HS, Falconer JR (May 2021). "Development and Optimization of Supercritical Fluid Extraction Setup Leading to Quantification of 11 Cannabinoids Derived from Medicinal Cannabis". Biology. 10 (6): 481. doi:10.3390/biology10060481. PMC 8227983. PMID 34071473.
- PubChem. "delta8-THC". pubchem.ncbi.nlm.nih.gov. Retrieved 9 November 2020.
- Commissioner, Office of the (4 May 2022). "5 Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8 THC". FDA.
- Razdan RK (1984). "Chemistry and Structure-Activity Relationships of Cannabinoids: An Overview". The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. pp. 63–78. doi:10.1016/b978-0-12-044620-9.50009-9. ISBN 978-0-12-044620-9.
- Hollister LE, Gillespie HK (May 1973). "Delta-8- and delta-9-tetrahydrocannabinol comparison in man by oral and intravenous administration". Clinical Pharmacology and Therapeutics. 14 (3): 353–7. doi:10.1002/cpt1973143353. PMID 4698563. S2CID 41556421.
- "delta-8-tetrahydrocannabinol". www.cancer.gov. 2 February 2011. Archived from the original on 24 April 2018. Retrieved 28 March 2021.
- "The Ultimate Guide to Delta 8 THC". Save On Cannabis - Cannabis Coupons and CBD Coupon Codes. 21 August 2020. Retrieved 30 May 2022.
- Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC (July 1973). "Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys". Toxicology and Applied Pharmacology. 25 (3): 363–72. doi:10.1016/0041-008x(73)90310-4. PMID 4199474.
- Chambers M. "ChemIDplus - 0005957755 - HCAWPGARWVBULJ-IAGOWNOFSA-N - delta-8-Tetrahydrocannabinol - Similar structures search, synonyms, formulas, resource links, and other chemical information". chem.nlm.nih.gov. Retrieved 13 June 2021.
- Adams MD, Earnhardt JT, Dewey WL, Harris LS (March 1976). "Vasoconstrictor actions of delta8- and delta9-tetrahydrocannabinol in the rat". The Journal of Pharmacology and Experimental Therapeutics. 196 (3): 649–56. PMID 4606.
- Richter JS, Quenardelle V, Rouyer O, Raul JS, Beaujeux R, Gény B, Wolff V (29 May 2018). "A Systematic Review of the Complex Effects of Cannabinoids on Cerebral and Peripheral Circulation in Animal Models". Frontiers in Physiology. 9: 622. doi:10.3389/fphys.2018.00622. PMC 5986896. PMID 29896112.
- Charalambous A, Marciniak G, Shiue CY, Dewey SL, Schlyer DJ, Wolf AP, Makriyannis A (November 1991). "PET studies in the primate brain and biodistribution in mice using (-)-5'-18F-delta 8-THC". Pharmacology, Biochemistry, and Behavior. 40 (3): 503–7. doi:10.1016/0091-3057(91)90354-5. PMID 1666914. S2CID 140208679.
- Tripathi HL, Vocci FJ, Brase DA, Dewey WL (1987). "Effects of cannabinoids on levels of acetylcholine and choline and on turnover rate of acetylcholine in various regions of the mouse brain". Alcohol and Drug Research. 7 (5–6): 525–32. PMID 3620017. INIST:7401152.
- Abrahamov A, Abrahamov A, Mechoulam R (May 1995). "An efficient new cannabinoid antiemetic in pediatric oncology". Life Sciences. 56 (23–24): 2097–102. doi:10.1016/0024-3205(95)00194-b. PMID 7776837.
- Walter L, Stella N (March 2004). "Cannabinoids and neuroinflammation". British Journal of Pharmacology. 141 (5): 775–85. doi:10.1038/sj.bjp.0705667. PMC 1574256. PMID 14757702.
- Morales P, Hurst DP, Reggio PH (2017). "Molecular Targets of the Phytocannabinoids: A Complex Picture". Phytocannabinoids. Progress in the Chemistry of Organic Natural Products. Vol. 103. Springer. pp. 103–131. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6. PMC 5345356. PMID 28120232.
- Bow EW, Rimoldi JM (January 2016). "The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry. 8: 17–39. doi:10.4137/PMC.S32171. PMC 4927043. PMID 27398024.
- Pertwee RG (January 2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin". British Journal of Pharmacology. 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC 2219532. PMID 17828291.
- Stout SM, Cimino NM (February 2014). "Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review". Drug Metabolism Reviews. 46 (1): 86–95. doi:10.3109/03602532.2013.849268. PMID 24160757. S2CID 29133059.
- Villamor JL, Bermejo AM, Tabernero MJ, Fernandez P, Sanchez I (December 1998). "GC/MS Determination of 11-Nor-9-Carboxy-Δ 8 -tetrahydrocannabinol in Urine from Cannabis Users". Analytical Letters. 31 (15): 2635–2643. doi:10.1080/00032719808005332.
- Valiveti S, Hammell DC, Earles DC, Stinchcomb AL (June 2005). "LC-MS method for the estimation of delta8-THC and 11-nor-delta8-THC-9-COOH in plasma". Journal of Pharmaceutical and Biomedical Analysis. 38 (1): 112–8. doi:10.1016/j.jpba.2004.11.055. PMID 15907628.
- Harvey DJ, Brown NK (November 1991). "Comparative in vitro metabolism of the cannabinoids". Pharmacology, Biochemistry, and Behavior. 40 (3): 533–40. doi:10.1016/0091-3057(91)90359-A. PMID 1806943. S2CID 25827210.
- Mechoulam R, BenZvi Z, Agurell S, Nilsson IM, Nilsson JL, Edery H, Grunfeld Y (October 1973). "Delta 6-tetrahydrocannabinol-7-oic acid, a urinary delta 6-THC metabolite: isolation and synthesis". Experientia. 29 (10): 1193–5. doi:10.1007/BF01935065. PMID 4758913. S2CID 27021897.
- Thomas BF, Compton DR, Martin BR (November 1990). "Characterization of the lipophilicity of natural and synthetic analogs of delta 9-tetrahydrocannabinol and its relationship to pharmacological potency". The Journal of Pharmacology and Experimental Therapeutics. 255 (2): 624–30. CiteSeerX 10.1.1.968.4912. PMID 2173751.
- Rosenkrantz H, Thompson GR, Braude MC (July 1972). "Oral and parenteral formulations of marijuana constituents". Journal of Pharmaceutical Sciences. 61 (7): 1106–12. doi:10.1002/jps.2600610715. PMID 4625586.
- Golombek P, Müller M, Barthlott I, Sproll C, Lachenmeier DW (June 2020). "Conversion of Cannabidiol (CBD) into Psychotropic Cannabinoids Including Tetrahydrocannabinol (THC): A Controversy in the Scientific Literature". Toxics. 8 (2): 41. doi:10.3390/toxics8020041. PMC 7357058. PMID 32503116.
- Gaoni Y, Mechoulam R (January 1966). "Hashish—VII". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- Marzullo P, Foschi F, Coppini DA, Fanchini F, Magnani L, Rusconi S, et al. (October 2020). "Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization". Journal of Natural Products. 83 (10): 2894–2901. doi:10.1021/acs.jnatprod.0c00436. PMC 8011986. PMID 32991167.
- "Is delta-8 THC safe? Here's what the experts say". Leafly. 9 June 2021. Retrieved 12 June 2021.
- Erickson BE (30 August 2021). "Delta-8-THC craze concerns chemists". Chemical & Engineering News. 99 (31).
- Potts AJ, Cano C, Thomas SH, Hill SL (February 2020). "Synthetic cannabinoid receptor agonists: classification and nomenclature". Clinical Toxicology. 58 (2): 82–98. doi:10.1080/15563650.2019.1661425. PMID 31524007. S2CID 202581071.
- Kelly BF, Nappe TM (2021). "Cannabinoid Toxicity". StatPearls. StatPearls Publishing. PMID 29489164.
- Laaris N, Good CH, Lupica CR (2010). "Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus". Neuropharmacology. 59 (1–2): 121–7. doi:10.1016/j.neuropharm.2010.04.013. PMC 2882293. PMID 20417220.
- Gamage TF, Barrus DG, Kevin RC, Finlay DB, Lefever TW, Patel PR, et al. (June 2020). "In vitro and in vivo pharmacological evaluation of the synthetic cannabinoid receptor agonist EG-018". Pharmacology, Biochemistry, and Behavior. 193: 172918. doi:10.1016/j.pbb.2020.172918. PMC 7239729. PMID 32247816.
- Huffman JW, Padgett LW (31 May 2005). "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry. 12 (12): 1395–411. doi:10.2174/0929867054020864. PMID 15974991.
- Adams R, Cain CK, McPhee WD, Wearn RB (August 1941). "Structure of Cannabidiol. XII. Isomerization to Tetrahydrocannabinols 1". Journal of the American Chemical Society. 63 (8): 2209–2213. doi:10.1021/ja01853a052.
- Adams R (November 1942). "Marihuana: Harvey Lecture, February 19, 1942". Bulletin of the New York Academy of Medicine. 18 (11): 705–30. PMC 1933888. PMID 19312292.
- Mechoulam R (June 1970). "Marihuana chemistry". Science. 168 (3936): 1159–66. Bibcode:1970Sci...168.1159M. doi:10.1126/science.168.3936.1159. PMID 4910003.
- Hively RL, Mosher WA, Hoffmann FW (April 1966). "Isolation of trans-delta-tetrahydrocannabinol from marijuana". Journal of the American Chemical Society. 88 (8): 1832–3. doi:10.1021/ja00960a056. PMID 5942992.
- Mechoulam R, Braun P, Gaoni Y (August 1967). "A stereospecific synthesis of (-)-delta 1- and (-)-delta 1(6)-tetrahydrocannabinols". Journal of the American Chemical Society. 89 (17): 4552–4. doi:10.1021/ja00993a072. PMID 6046550.
- Pertwee RG (January 2006). "Cannabinoid pharmacology: the first 66 years". British Journal of Pharmacology. 147 (Suppl 1): S163-71. doi:10.1038/sj.bjp.0706406. PMC 1760722. PMID 16402100.
- Amanda Reed (13 December 2021). "What the Hell Is Delta-8 THC?". Gear Patrol. Retrieved 8 August 2022.
- "CBD: A Legal High… or Not? What Is Delta-8? Local Cannabinologist Weig". Tennessee Farmaceuticals. Retrieved 28 February 2022.
- iHemp. "What Is A Vape Pen?". iHemp. Retrieved 19 September 2021.
- Farah T (23 September 2020). "Delta-8-THC Promises to Get You High Without the Paranoia or Anxiety". Discover Magazine. Retrieved 9 November 2020.
- Levey L (23 October 2020). "DEA Tried Banning New Cannabis Product, Sellers Still Selling". Dope Magazine. Retrieved 10 November 2020.
- King S (18 January 2021). "How Some THC Is Legal — For Now". Rolling Stone.
- Richtel M (27 February 2021). "This Drug Gets You High, and Is Legal (Maybe) Across the Country". The New York Times. Archived from the original on 27 February 2021. Retrieved 28 March 2021.
- "The Unregulated Distribution And Sale Of Consumer Products Marketed As Delta-8 THC" (PDF). US Cannabis Council. Archived from the original on 4 July 2022. Retrieved 31 March 2022.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - Sachs, Jenna (20 December 2021). "CBD store owner faces felony charges after raid". FOX6 News Milwaukee. Retrieved 31 March 2022.
- "GSO hemp shop owners say police wrongly searched, seized legal THC products". Triad City Beat. 27 October 2021. Retrieved 31 March 2022.
- Pelkey, Scott (28 March 2022). "Asheboro PD VICE/Narcotics Team Cracks Down on Illegal THC Devices At Local Vape Shops". Randolph News Now. Retrieved 31 March 2022.
- Maggiore, Sabrina (14 March 2022). "Sheriff to Catoosa County smoke shop owners: Your Delta 8 products violate state law". WFLI. Retrieved 31 March 2022.
- "Korova Korova - Biggie Cookie, 1,000mg Reviews | Weedmaps". weedmaps.com. Archived from the original on 16 April 2022. Retrieved 19 April 2022.
- "Delta-8 THC: The lesser known cannabinoid that FarmaceuticalRX is taking on — Maitri Medicinals". www.maitrimedicinals.com. Archived from the original on 16 April 2022. Retrieved 19 April 2022.
- "Archived copy". preview.redd.it. Archived from the original on 16 April 2022. Retrieved 19 April 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - "ENROLLED HOUSE BILL No. 4517". Bill of 13 July 2021. Michigan Legislature. Archived from the original on 23 September 2022. Retrieved 22 September 2022.
- "House Bill 3000". Bill of 19 July 2021. Oregon Legislative Assembly. Archived from the original on 1 July 2022. Retrieved 22 September 2022.
- Mike (16 August 2022). "9th Circuit Finds Delta 8 THC Legal". DELTA GUMMIES. Retrieved 12 September 2022.
- Britt E. Erickson (29 August 2021). "Delta-8-THC craze concerns chemists: Unidentified by-products and lack of regulatory oversight spell trouble for cannabis products synthesized from CBD". Chemical & Engineering News.
- Commissioner, Office of the (25 March 2022). "5 Things to Know about Delta-8 Tetrahydrocannabinol – Delta-8 THC". FDA.
- Punyamurthula NS, Adelli GR, Gul W, Repka MA, ElSohly MA, Majumdar S (August 2017). "Ocular Disposition of ∆8-Tetrahydrocannabinol from Various Topical Ophthalmic Formulations". AAPS PharmSciTech. 18 (6): 1936–1945. doi:10.1208/s12249-016-0672-2. PMC 5629008. PMID 27905004.
- Muchtar S, Almog S, Torracca MT, Saettone MF, Benita S (1992). "A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: effect on intraocular pressure in rabbits". Ophthalmic Research. 24 (3): 142–9. doi:10.1159/000267160. PMID 1328979.
- Thapa D, Cairns EA, Szczesniak AM, Kulkarni PM, Straiker AJ, Thakur GA, Kelly ME (January 2020). "Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation". Molecules. 25 (2): 417. doi:10.3390/molecules25020417. PMC 7024337. PMID 31968549.
- Thapa D, Cairns EA, Szczesniak AM, Toguri JT, Caldwell MD, Kelly ME (May 2018). "The Cannabinoids Δ8THC, CBD, and HU-308 Act via Distinct Receptors to Reduce Corneal Pain and Inflammation". Cannabis and Cannabinoid Research. 3 (1): 11–20. doi:10.1089/can.2017.0041. PMC 5812319. PMID 29450258.