JWH-161
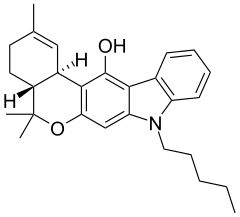
JWH-161 is a cannabinoid derivative that was designed by Dr John W. Huffman's team as a hybrid between the dibenzopyran "classical" cannabinoid drugs and the novel indole derivatives, in an attempt to unravel the differences in their binding modes to the CB1 receptor. While retaining structural elements from both families, JWH-161 has a CB1 Ki of 19.0nM, although it was found to be slightly weaker than THC in animal tests.[1]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C27H33NO2 |
| Molar mass | 403.566 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
References
- Huffman JW, Padgett LW (2005). "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry. 12 (12): 1395–411. doi:10.2174/0929867054020864. PMID 15974991.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.