List of JWH cannabinoids
The John W. Huffman research group at Clemson University synthesized over 450 cannabinoids.[1][2][3][4] Some of those are:
| Name | Class | Ki / nM at CB1 | Ki / nM at CB2 | Selectivity | Structure |
|---|---|---|---|---|---|
| JWH-004 | Naphthoylindole | 48 ± 13 | 4 ± 1.5 | CB2 (12x) | 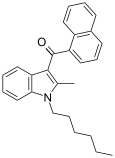 |
| JWH-007[5] | Naphthoylindole | 9.5 ± 4.5 | 2.9 ± 2.6 | CB2 (3.3x) |  |
| JWH-009 | Naphthoylindole | >10000 | 141 ± 14 | CB2 (>70x) |  |
| JWH-011 | Naphthoylindole |  | |||
| JWH-015[5] | Naphthoylindole | 164 ± 22 | 13.8 ± 4.6 | CB2 (12x) |  |
| JWH-016 | Naphthoylindole | 22 ± 1.5 | 4.3 ± 1.6 | CB2 (5.1x) |  |
| JWH-018[5] | Naphthoylindole | 9 ± 5 | 2.9 ± 2.6 | CB2 (3.1x) |  |
| JWH-019 | Naphthoylindole | 9.8 ± 2 | 5.55 ± 2 | CB2 (1.77x) | 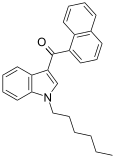 |
| JWH-020 | Naphthoylindole | 128 ± 17 | 205 ± 20 | CB1 (1.6x) | 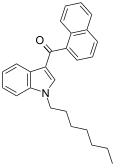 |
| JWH-030 | Naphthoylpyrrole | 87 ± 3 | 320 ± 127 | CB1 (3.7x) |  |
| JWH-031 | Naphthoylpyrrole | 399 ± 109 |  | ||
| JWH-032 | Naphthoylpyrrole | >10000 | >10000 | — |  |
| JWH-033 | Naphthoylpyrrole | 666 ± 77 | 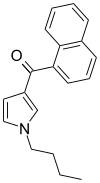 | ||
| JWH-036 | Naphthoylpyrrole | 309 ± 11 | 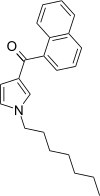 | ||
| JWH-042[6] | Naphthoylindole | >10000 | 5050 ± 192 | CB2 |  |
| JWH-043[6] | Naphthoylindole | 1180 ± 44 | 964 ± 242 | CB2 (1.2x) |  |
| JWH-044 | Naphthoylpyrrole | >10000 | >10000 | — | 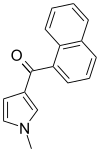 |
| JWH-045 | Naphthoylpyrrole | >10000 | >10000 | — |  |
| JWH-046[6] | Naphthoylindole | 343 ± 38 | 16.3 ± 4.9 | CB2 (21x) | 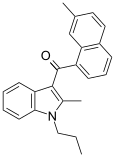 |
| JWH-047[6] | Naphthoylindole | 59 ± 3 | 3.47 ± 1.80 | CB2 (17x) | 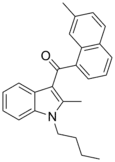 |
| JWH-048[6] | Naphthoylindole | 10.7 ± 1.0 | 0.49 ± 0.13 | CB2 (22x) |  |
| JWH-049[6] | Naphthoylindole | 55.1 ± 17.0 | 32.3 ± 2.4 | CB2 (1.7x) |  |
| JWH-050[6] | Naphthoylindole | 342 ± 6 | 526 ± 133 | CB1 (1.5x) | 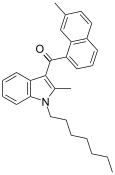 |
| JWH-051 | Dibenzopyran | 1.20 | 0.03 | CB2 (40x) |  |
| JWH-056[7] | Dibenzopyran | >10000 | 32 ± 9 | CB2 |  |
| JWH-057[8] | Dibenzopyran | 23 ± 7 | 2.9 ± 1.6 | CB2 (8x) |  |
| JWH-065[7] | Dibenzopyran | 399 ± 76 | 10 ± 2 | CB2 (40x) | 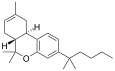 |
| JWH-070[6] | Naphthoylindole | >10000 | >10000 | 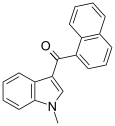 | |
| JWH-071[6] | Naphthoylindole | 1340 ± 123 | 2940 ± 852 | CB1 (2.2x) | 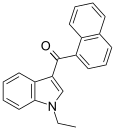 |
| JWH-072 | Naphthoylindole | 1050 ± 5.5 | 170 ± 54 | CB2 (6x) | 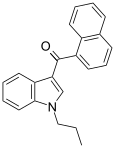 |
| JWH-073 | Naphthoylindole | 8.9 ± 1.8 | 27 ± 12 | CB1 (3x) | 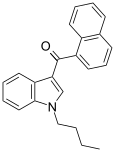 |
| JWH-076[5] | Naphthoylindole | 214 ± 11 | 106 ± 46 | CB2 (2x) | 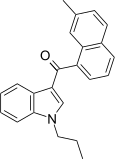 |
| JWH-077[6] | Naphthoylindole | >10000 | >10000 |  | |
| JWH-078[6] | Naphthoylindole | 817 ± 60 | 633 ± 116 | CB2 (1.3x) | 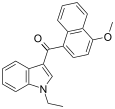 |
| JWH-079[6] | Naphthoylindole | 63.0 ± 3.0 | 32.0 ± 6.0 | CB2 (2x) | 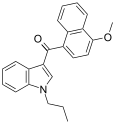 |
| JWH-080[6] | Naphthoylindole | 8.9 ± 1.8 | 2.21 ± 1.30 | CB2 (4x) | 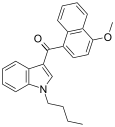 |
| JWH-081[6] | Naphthoylindole | 1.2 ± 0.03 | 12.4 ± 2.2 | CB1 (10x) | 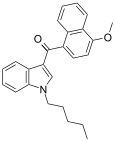 |
| JWH-082[6] | Naphthoylindole | 5.3 ± 0.8 | 6.40 ± 0.94 | CB1 (1.2x) |  |
| JWH-083[6] | Naphthoylindole | 106 ± 12 | 102 ± 50 | — |  |
| JWH-091[9] (Δ8-THCP) | Dibenzopyran | 22.0 ± 3.9 |  | ||
| JWH-093[6] | Naphthoylindole | 40.7 ± 2.8 | 59.1 ± 10.5 | CB1 (1.45x) | 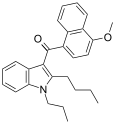 |
| JWH-094[6] | Naphthoylindole | 476 ± 67 | 97.3 ± 2.7 | CB2 (4.9x) |  |
| JWH-095[6] | Naphthoylindole | 140 ± 4.3 | 312 ± 83 | CB1 (2.2x) |  |
| JWH-096[6] | Naphthoylindole | 33.7 ± 2.9 | 13.3 ± 5.6 | CB2 (2.5x) |  |
| JWH-097[6] | Naphthoylindole | 455 ± 28 | 121 ± 15 | CB2 (3.8x) |  |
| JWH-098[6] | Naphthoylindole | 4.5 ± 0.1 | 1.9 ± 0.3 | CB2 (2.4x) | 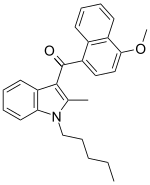 |
| JWH-099[6] | Naphthoylindole | 35.3 ± 9.0 | 17.8 ± 2.9 | CB2 (2x) |  |
| JWH-100[6] | Naphthoylindole | 381 ± 102 | 155 ± 74 | CB2 (2.5x) |  |
| JWH-102[7] | Dibenzopyran | 7.9 ± 0.9 | 5.2 ± 2.0 | CB2 (1.5x) |  |
| JWH-103[7] | Dibenzopyran | 28 ± 3 | 23 ± 7 | CB2 (1.2x) |  |
| JWH-116[10] | Naphthoylindole | 52 ± 5 |  | ||
| JWH-120[5] | Naphthoylindole | 1054 ± 31 | 6.1 ± 0.7 | CB2 (173x) |  |
| JWH-122[10] | Naphthoylindole | 0.69 ± 0.05 | 1.2 ± 1.2 | — | 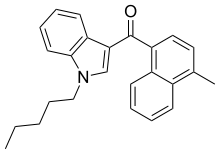 |
| JWH-124 (Δ8-Parahexyl) | Dibenzopyran | 41.0 ± 3.8 |  | ||
| JWH-130 (Δ8-THCB) | Dibenzopyran | 65.0 ± 13 |  | ||
| JWH-133[7] | Dibenzopyran | 677 ± 132 | 3.4 ± 1.0 | CB2 (200x) |  |
| JWH-138[11] | Dibenzopyran | 8.5 ± 1.4 |  | ||
| JWH-139[12] | Dibenzopyran | 2290 ± 505 | 14 ± 10 | CB2 (164x) | 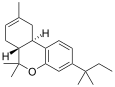 |
| JWH-142[7] | Dibenzopyran | 529 ± 49 | 35 ± 14 | CB2 (15x) | 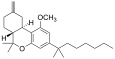 |
| JWH-143[7] | Dibenzopyran | 924 ± 104 | 65 ± 8 | CB2 (14x) | 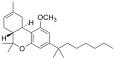 |
| JWH-145[13] | Naphthoylpyrrole | 14 ± 2 | 6.4 ± 0.4 | CB2 (2.2x) |  |
| JWH-146[13] | Naphthoylpyrrole | 21 ± 2 | 62 ± 5 | CB2 (3.0x) |  |
| JWH-147[13] | Naphthoylpyrrole | 11 ± 1 | 7.1 ± 0.2 | CB2 (1.5x) |  |
| JWH-148[5] | Naphthoylindole | 123 ± 8 | 14.0 ± 1.0 | CB2 (8x) | 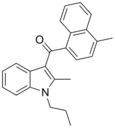 |
| JWH-149[5] | Naphthoylindole | 5.0 ± 2.1 | 0.73 ± 0.03 | CB2 (6.8x) |  |
| JWH-150[13] | Naphthoylpyrrole | 60 ± 1 | 15 ± 2 | CB2 (4x) | 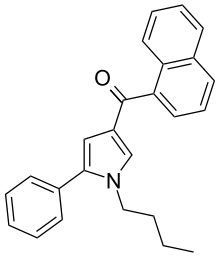 |
| JWH-151[5] | Naphthoylindole | >10000 | 30 ± 1.1 | CB2 (>333x) | 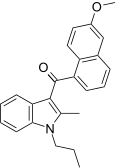 |
| JWH-153[5] | Naphthoylindole | 250 ± 24 | 11 ± 0.5 | CB2 (23x) |  |
| JWH-156[13] | Naphthoylpyrrole | 404 ± 18 | 104 ± 18 | CB2 (4x) | 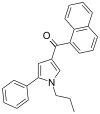 |
| JWH-159[5] | Naphthoylindole | 45 ± 1 | 10.4 ± 1.4 | CB2 (4.3x) | 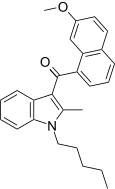 |
| JWH-160[5] | Naphthoylindole | 1568 ± 201 | 441 ± 110 | CB2 (3.6x) | 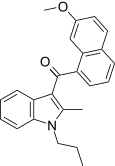 |
| JWH-161 | Dibenzopyran hybrid | 19.0 | 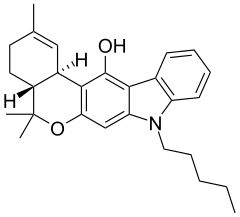 | ||
| JWH-163[5] | Naphthoylindole | 2358 ± 215 | 138 ± 12 | CB2 (17x) | 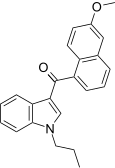 |
| JWH-164[5] | Naphthoylindole | 6.6 ± 0.7 | 6.9 ± 0.2 | — |  |
| JWH-165[5] | Naphthoylindole | 204 ± 26 | 71 ± 8 | CB2 (2.9x) |  |
| JWH-166[5] | Naphthoylindole | 44 ± 10 | 1.9 ± 0.08 | CB2 (23x) |  |
| JWH-167 | Phenylacetylindole | 90 ± 17 | 159 ± 14 | CB1 (1.77x) |  |
| JWH-171 | Hydrocarbon | 51 |  | ||
| JWH-175[10] | Naphthylmethylindole | 22 ± 2 |  | ||
| JWH-176[10] | Hydrocarbon | 26 ± 4 | 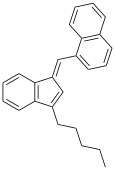 | ||
| JWH-180[5] | Naphthoylindole | 26 ± 2 | 9.6 ± 2.0 | CB2 (2.7x) |  |
| JWH-181[5] | Naphthoylindole | 1.3 ± 0.1 | 0.62 ± 0.04 | CB2 (2.1x) | 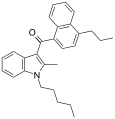 |
| JWH-182[5] | Naphthoylindole | 0.65 ± 0.03 | 1.1 ± 0.1 | CB1 (1.7x) |  |
| JWH-184[10] | Naphthylmethylindole | 23 ± 6 | 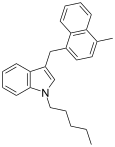 | ||
| JWH-185[10] | Naphthylmethylindole | 17 ± 3 | 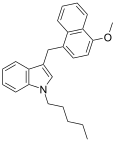 | ||
| JWH-186[14] | Dibenzopyran | 187 ± 23 | 5.6 ± 1.7 | CB2 (33x) |  |
| JWH-187[14] | Dibenzopyran | 84 ± 16 | 3.4 ± 0.5 | CB2 (25x) |  |
| JWH-188[14] | Dibenzopyran | 270 ± 58 | 18 ± 2 | CB2 (15x) | 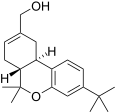 |
| JWH-189[5] | Naphthoylindole | 52 ± 2 | 12 ± 0.8 | CB2 (4.3x) | 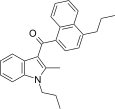 |
| JWH-190[14] | Dibenzopyran | 8.8 ± 1.4 | 1.6 ± 0.03 | CB2 (5.5x) | 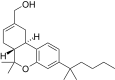 |
| JWH-191[14] | Dibenzopyran | 1.8 ± 0.3 | 0.52 ± 0.03 | CB2 (3.5x) | 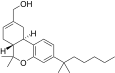 |
| JWH-192[10] | Naphthylmethylindole | 41 ± 13 |  | ||
| JWH-193[10] | Naphthoylindole | 6 ± 1 |  | ||
| JWH-194[10] | Naphthylmethylindole | 127 ± 19 | 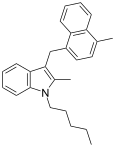 | ||
| JWH-195[10] | Naphthylmethylindole | 113 ± 28 |  | ||
| JWH-196[10] | Naphthylmethylindole | 151 ± 18 |  | ||
| JWH-197[10] | Naphthylmethylindole | 323 ± 98 |  | ||
| JWH-198[10] | Naphthoylindole | 10 ± 2 |  | ||
| JWH-199[10] | Naphthylmethylindole | 20 ± 2 | 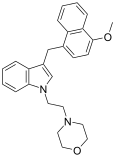 | ||
| JWH-200[10] | Naphthoylindole | 42 ± 5 |  | ||
| JWH-201[15] | Phenylacetylindole | 1064 ± 21 | 444 ± 14 | CB2 (2.4x) |  |
| JWH-202[15] | Phenylacetylindole | 1678 ± 63 | 645 ± 6 | CB2 (2.6x) |  |
| JWH-203[15] | Phenylacetylindole | 8.0 ± 0.9 | 7.0 ± 1.3 | — |  |
| JWH-204[15] | Phenylacetylindole | 13 ± 1 | 25 ± 1 | CB1 (1.9x) | 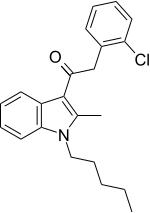 |
| JWH-205[15] | Phenylacetylindole | 124 ± 23 | 180 ± 9 | CB1 (1.45x) | 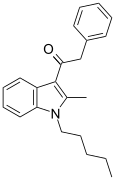 |
| JWH-206[15] | Phenylacetylindole | 389 ± 25 | 498 ± 37 | CB1 (1.28x) | 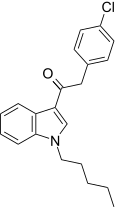 |
| JWH-207[15] | Phenylacetylindole | 1598 ± 134 | 3723 ± 10 | CB1 (2.33x) |  |
| JWH-208[15] | Phenylacetylindole | 179 ± 7 | 570 ± 127 | CB1 (3.18x) |  |
| JWH-209[15] | Phenylacetylindole | 746 ± 49 | 1353 ± 270 | CB1 (1.81x) |  |
| JWH-210[5] | Naphthoylindole | 0.46 ± 0.03 | 0.69 ± 0.01 | CB1 (1.5x) | 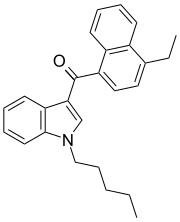 |
| JWH-211[5] | Naphthoylindole | 70 ± 0.8 | 12 ± 0.8 | CB2 (5.8x) |  |
| JWH-212[5] | Naphthoylindole | 33 ± 0.9 | 10 ± 1.2 | CB2 (3.3x) | 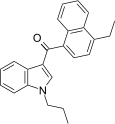 |
| JWH-213[5] | Naphthoylindole | 1.5 ± 0.2 | 0.42 ± 0.05 | CB2 (3.6x) |  |
| JWH-215[14] | Dibenzopyran | 1008 ± 117 | 85 ± 21 | CB2 (12x) | 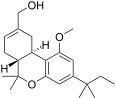 |
| JWH-216[14] | Dibenzopyran | 1856 ± 148 | 333 ± 104 | CB2 (5.6x) |  |
| JWH-217[14] | Dibenzopyran | >10000 | 1404 ± 66 | CB2 (>7x) |  |
| JWH-220 | Hydrocarbon | 19 | 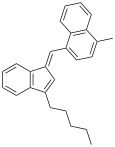 | ||
| JWH-224[14] | Dibenzopyran | 347 ± 34 | 28 ± 1 | CB2 (12.3x) |  |
| JWH-225[14] | Dibenzopyran | >10000 | 325 ± 70 | CB2 (>31x) |  |
| JWH-226[14] | Dibenzopyran | 4001 ± 282 | 43 ± 3 | CB2 (93x) |  |
| JWH-227[14] | Dibenzopyran | 40 ± 6 | 4.4 ± 0.3 | CB2 (9x) | 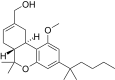 |
| JWH-229[16] | Dibenzopyran | 3134 ± 110 | 18 ± 2 | CB2 (174x) | 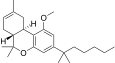 |
| JWH-230[14] | Dibenzopyran | 15 ± 3 | 1.4 ± 0.12 | CB2 (10.7x) | 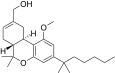 |
| JWH-233[14] | Dibenzopyran | 14 ± 3 | 1.0 ± 0.3 | CB2 (14x) |  |
| JWH-234[5] | Naphthoylindole | 8.4 ± 1.8 | 3.8 ± 0.6 | CB2 (2.2x) | 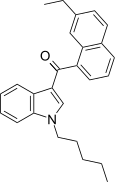 |
| JWH-235[5] | Naphthoylindole | 338 ± 34 | 123 ± 34 | CB2 (2.7x) |  |
| JWH-236[5] | Naphthoylindole | 1351 ± 204 | 240 ± 63 | CB2 (5.6x) |  |
| JWH-237[15] | Phenylacetylindole | 38 ± 10 | 106 ± 2 | CB1 (2.8x) | 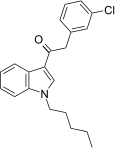 |
| JWH-239[5] | Naphthoylindole | 342 ± 20 | 52 ± 6 | CB2 (6.6x) | 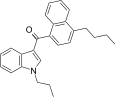 |
| JWH-240[5] | Naphthoylindole | 14 ± 1 | 7.2 ± 1.3 | CB2 (1.9x) | 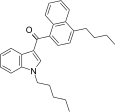 |
| JWH-241[5] | Naphthoylindole | 147 ± 20 | 49 ± 7 | CB2 (3.0x) | 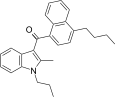 |
| JWH-242[5] | Naphthoylindole | 42 ± 9 | 6.5 ± 0.3 | CB2 (6.5x) |  |
| JWH-243[13] | Naphthoylpyrrole | 285 ± 40 | 41 ± 3 | CB2 (6.95x) | 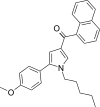 |
| JWH-244[13] | Naphthoylpyrrole | 130 ± 6 | 18 ± 1 | CB2 (7.22x) | 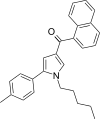 |
| JWH-245[13] | Naphthoylpyrrole | 276 ± 4 | 25 ± 2 | CB2 (11x) |  |
| JWH-246[13] | Naphthoylpyrrole | 70 ± 4 | 16 ± 1 | CB2 (4.38x) |  |
| JWH-247[14] | Dibenzopyran | 427 ± 31 | 99 ± 4 | CB2 (4.3x) |  |
| JWH-248[15] | Phenylacetylindole | 1028 ± 39 | 657 ± 19 | CB2 (1.56x) |  |
| JWH-249[15] | Phenylacetylindole | 8.4 ± 1.8 | 20 ± 2 | CB1 (2.38x) | 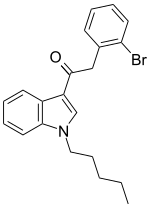 |
| JWH-250[15] | Phenylacetylindole | 11 ± 2 | 33 ± 2 | CB1 (3x) |  |
| JWH-251[15] | Phenylacetylindole | 29 ± 3 | 146 ± 36 | CB2 (5x) | 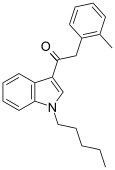 |
| JWH-252[15] | Phenylacetylindole | 23 ± 3 | 19 ± 1 | CB2 (1.2x) |  |
| JWH-253[15] | Phenylacetylindole | 62 ± 10 | 84 ± 12 | CB1 (1.35x) | 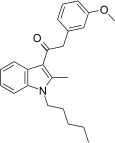 |
| JWH-254[14] | Dibenzopyran | 4724 ± 509 | 319 ± 16 | CB2 (14.8x) | 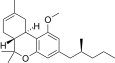 |
| JWH-256[14] | Dibenzopyran | 4300 ± 888 | 97 ± 18 | CB2 (44x) |  |
| JWH-258[5] | Naphthoylindole | 4.6 ± 0.6 | 10.5 ± 1.3 | CB1 (2.3x) |  |
| JWH-259[5] | Naphthoylindole | 220 ± 29 | 74 ± 7 | CB2 (3.0x) | 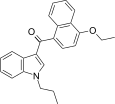 |
| JWH-260[5] | Naphthoylindole | 29 ± 0.4 | 25 ± 1.9 | CB2 (1.2x) |  |
| JWH-261[5] | Naphthoylindole | 767 ± 105 | 221 ± 14 | CB2 (3.5x) |  |
| JWH-262[5] | Naphthoylindole | 28 ± 3 | 5.6 ± 0.7 | CB2 (5.0x) |  |
| JWH-265[5] | Naphthoylindole | 3788 ± 323 | 80 ± 13 | CB2 (47x) |  |
| JWH-266[5] | Naphthoylindole | >10000 | 455 ± 55 | CB2 (>22x) |  |
| JWH-267[5] | Naphthoylindole | 381 ± 16 | 7.2 ± 0.14 | CB2 (53x) |  |
| JWH-268[5] | Naphthoylindole | 1379 ± 193 | 40 ± 0.6 | CB2 (34x) |  |
| JWH-277[14] | Dibenzopyran | 3905 ± 91 | 589 ± 65 | CB2 (6.6x) |  |
| JWH-278[14] | Dibenzopyran | 906 ± 80 | 69 ± 6 | CB2 (13x) |  |
| JWH-292[13] | Naphthoylpyrrole | 29 ± 1 | 20 ± 1 | CB2 (1.45x) |  |
| JWH-293[13] | Naphthoylpyrrole | 100 ± 5 | 41 ± 4 | CB2 (2.44x) |  |
| JWH-298[14] | Dibenzopyran | 812 ± 67 | 198 ± 23 | CB2 (4.1x) |  |
| JWH-299[14] | Dibenzopyran | 415 ± 50 | 30 ± 2 | CB2 (13.8x) | 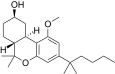 |
| JWH-300[12] | Dibenzopyran | 118 ± 16 | 5.3 ± 0.1 | CB2 (22x) |  |
| JWH-301[14] | Dibenzopyran | 295 ± 64 | 48 ± 4 | CB2 (6.1x) | 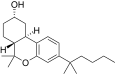 |
| JWH-302[15] | Phenylacetylindole | 17 ± 2 | 89 ± 15 | CB1 (5.26x) |  |
| JWH-303[15] | Phenylacetylindole | 117 ± 10 | 138 ± 12 | CB1 (1.18x) |  |
| JWH-304[15] | Phenylacetylindole | 3363 ± 332 | 2679 ± 688 | CB2 (1.26x) |  |
| JWH-305[15] | Phenylacetylindole | 15 ± 1.8 | 29 ± 5 | CB1 (1.93x) | 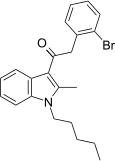 |
| JWH-306[15] | Phenylacetylindole | 25 ± 1 | 82 ± 11 | CB1 (3.28x) |  |
| JWH-307[13] | Naphthoylpyrrole | 7.7 ± 1.8 | 3.3 ± 0.2 | CB2 (2.33x) | 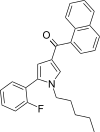 |
| JWH-308[13] | Naphthoylpyrrole | 41 ± 1 | 33 ± 2 | CB2 (1.24x) |  |
| JWH-309[13] | Naphthoylpyrrole | 41 ± 3 | 49 ± 7 | CB1 (1.20x) | 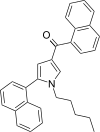 |
| JWH-310[14] | Dibenzopyran | 1059 ± 51 | 36 ± 3 | CB2 (29x) |  |
| JWH-311[15] | Phenylacetylindole | 23 ± 2 | 39 ± 3 | CB1 (1.70x) | 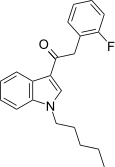 |
| JWH-312[15] | Phenylacetylindole | 72 ± 7 | 91 ± 20 | CB1 (1.26x) |  |
| JWH-313[15] | Phenylacetylindole | 422 ± 19 | 365 ± 92 | CB2 (1.16x) |  |
| JWH-314[15] | Phenylacetylindole | 39 ± 2 | 76 ± 4 | CB1 (1.95x) |  |
| JWH-315[15] | Phenylacetylindole | 430 ± 24 | 182 ± 23 | CB2 (3.36x) | 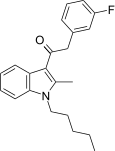 |
| JWH-316[15] | Phenylacetylindole | 2862 ± 670 | 781 ± 105 | CB2 (3.66x) | 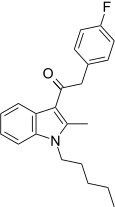 |
| JWH-336[12] | Dibenzopyran | 4589 ± 367 | 153 ± 15 | CB2 (30x) |  |
| JWH-338[14] | Dibenzopyran | >10000 | 111 ± 16 | CB2 (>90x) | 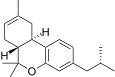 |
| JWH-339[14] | Dibenzopyran | >10000 | 2317 ± 93 | CB2 (>4.3x) |  |
| JWH-340[14] | Dibenzopyran | 135 ± 6 | 30 ± 1 | CB2 (4.5x) |  |
| JWH-341[14] | Dibenzopyran | 100 ± 8 | 10 ± 0.1 | CB2 (10x) |  |
| JWH-346[13] | Naphthoylpyrrole | 67 ± 6 | 39 ± 2 | CB2 (1.72x) |  |
| JWH-347[13] | Naphthoylpyrrole | 333 ± 17 | 169 ± 17 | CB2 (1.97x) | 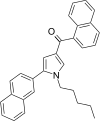 |
| JWH-348[13] | Naphthoylpyrrole | 218 ± 19 | 53 ± 1 | CB2 (4.11x) |  |
| JWH-349[14] | Dibenzopyran | 376 ± 1 | 38 ± 4 | CB2 (9.9x) | 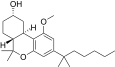 |
| JWH-350[12] | Dibenzopyran | 395 ± 50 | 12 ± 1 | CB2 (33x) | 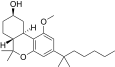 |
| JWH-351[14] | Dibenzopyran | >10000 | 295 ± 3 | CB2 (>34x) | 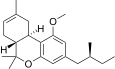 |
| JWH-352[14] | Dibenzopyran | >10000 | 47 ± 2 | CB2 (>213x) |  |
| JWH-353[14] | Dibenzopyran | 1493 ± 10 | 31 ± 1 | CB2 (48x) | 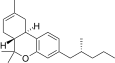 |
| JWH-354[14] | Dibenzopyran | 1961 ± 21 | 241 ± 14 | CB2 (8.1x) |  |
| JWH-355[14] | Dibenzopyran | 2162 ± 220 | 108 ± 17 | CB2 (20x) |  |
| JWH-356[14] | Dibenzopyran | 5837 ± 701 | 108 ± 17 | CB2 (54x) |  |
| JWH-357[14] | Dibenzopyran | 647 ± 78 | 185 ± 4 | CB2 (3.5x) | 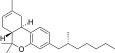 |
| JWH-358[14] | Dibenzopyran | 1243 ± 266 | 52 ± 3 | CB2 (24x) | 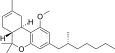 |
| JWH-359 | Dibenzopyran | 2918 ± 450 | 13.0 ± 0.2 | CB2 (220x) |  |
| JWH-360[14] | Dibenzopyran | 2449 ± 606 | 160 ± 8 | CB2 (15x) |  |
| JWH-361[14] | Dibenzopyran | 63 ± 3 | 2.7 ± 0.1 | CB2 (23x) |  |
| JWH-362[14] | Dibenzopyran | 127 ± 8 | 34 ± 5 | CB2 (3.7x) |  |
| JWH-363[13] | Naphthoylpyrrole | 245 ± 5 | 71 ± 1 | CB2 (3.45x) |  |
| JWH-364[13] | Naphthoylpyrrole | 34 ± 3 | 29 ± 1 | CB2 (1.17x) | 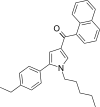 |
| JWH-365[13] | Naphthoylpyrrole | 17 ± 1 | 3.4 ± 0.2 | CB2 (5.0x) |  |
| JWH-366[13] | Naphthoylpyrrole | 191 ± 12 | 24 ± 1 | CB2 (7.96x) |  |
| JWH-367[13] | Naphthoylpyrrole | 53 ± 2 | 23 ± 1 | CB2 (2.30x) |  |
| JWH-368[13] | Naphthoylpyrrole | 16 ± 1 | 9.1 ± 0.7 | CB2 (1.76x) |  |
| JWH-369[13] | Naphthoylpyrrole | 7.9 ± 0.4 | 5.2 ± 0.3 | CB2 (1.52x) | 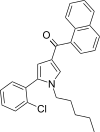 |
| JWH-370[13] | Naphthoylpyrrole | 5.6 ± 0.4 | 4.0 ± 0.5 | CB2 (1.40x) |  |
| JWH-371[13] | Naphthoylpyrrole | 42 ± 1 | 64 ± 2 | CB1 (1.52x) | 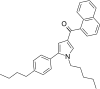 |
| JWH-372[13] | Naphthoylpyrrole | 77 ± 2 | 8.2 ± 0.2 | CB1 (9.39x) |  |
| JWH-373[13] | Naphthoylpyrrole | 60 ± 3 | 69 ± 2 | CB1 (1.15x) | 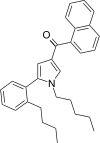 |
| JWH-387[17] | Naphthoylindole | 1.2 ± 0.1 | 1.1 ± 0.1 | — |  |
| JWH-398[18] | Naphthoylindole | 2.3 ± 0.1 | 2.8 ± 0.2 | CB1 (1.22x) | |
| JWH-416[17] | Naphthoylindole | 73 ± 10 | 3.3 ± 0.1 | CB2 (22x) |  |
| JWH-417[17] | Naphthoylindole | 522 ± 58 | 13 ± 0.2 | CB2 (40x) |  |
| JWH-422[17] | Naphthoylindole | 501 ± 48 | 20 ± 0.4 | CB2 (25x) |  |
| JWH-423[17] | Naphthoylindole | 140 ± 10 | 6.6 ± 0.2 | CB2 (21x) |  |
| JWH-424[17] | Naphthoylindole | 21 ± 3.4 | 5.4 ± 0.2 | CB2 (3.9x) |  |
| JWH-425[17] | Naphthoylindole | 54 ± 11 | 10 ± 0.4 | CB2 (5.4x) | 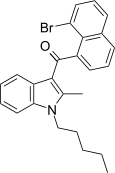 |
See also
Notes
- Ki is the compound's binding affinity for the cannabinoid receptor type 1 (CB1) or cannabinoid receptor type 2 (CB2).
References
- Manera C, Tuccinardi T, Martinelli A (2008). "Indoles and related compounds as cannabinoid ligands". Mini Rev Med Chem. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- Wiley JL, Marusich JA, Huffman JW (2014). "Moving around the molecule: relationship between chemical structure and in vivo activity of synthetic cannabinoids". Life Sci. 97 (1): 55–63. doi:10.1016/j.lfs.2013.09.011. PMC 3944940. PMID 24071522.
- Wiley JL, Marusich JA, Thomas BF (2017). "Combination Chemistry: Structure-Activity Relationships of Novel Psychoactive Cannabinoids". Curr Top Behav Neurosci. Current Topics in Behavioral Neurosciences. 32: 231–248. doi:10.1007/7854_2016_17. ISBN 978-3-319-52442-9. PMID 27753007.
- Banister SD, Connor M (2018). "The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonists as New Psychoactive Substances: Origins". Handb Exp Pharmacol. Handbook of Experimental Pharmacology. 252: 165–190. doi:10.1007/164_2018_143. ISBN 978-3-030-10560-0. PMID 29980914.
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson AL, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR (January 2005). "Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists". Bioorganic & Medicinal Chemistry. 13 (1): 89–112. doi:10.1016/j.bmc.2004.09.050. PMID 15582455.
- Aung MM, Griffin G, Huffman JW, Wu M, Keel C, Yang B, Showalter VM, Abood ME, Martin BR (August 2000). "Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding". Drug and Alcohol Dependence. 60 (2): 133–40. doi:10.1016/S0376-8716(99)00152-0. PMID 10940540.
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR (December 1999). "3-(1',1'-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor". Bioorganic & Medicinal Chemistry. 7 (12): 2905–14. doi:10.1016/s0968-0896(99)00219-9. PMID 10658595.
- Huffman JW, Yu S, Showalter V, Abood ME, Wiley JL, Compton DR, Martin BR, Bramblett RD, Reggio PH (September 1996). "Synthesis and pharmacology of a very potent cannabinoid lacking a phenolic hydroxyl with high affinity for the CB2 receptor". Journal of Medicinal Chemistry. 39 (20): 3875–7. doi:10.1021/JM960394Y. PMID 8831752.
- Bow EW, Rimoldi JM. The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect Medicin Chem. 2016 Jun 28;8:17-39. doi:10.4137/PMC.S32171 PMID 27398024
- Huffman JW, Mabon R, Wu MJ, Lu J, Hart R, Hurst DP, Reggio PH, Wiley JL, Martin BR (February 2003). "3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB(1) cannabinoid receptor". Bioorganic & Medicinal Chemistry. 11 (4): 539–49. doi:10.1016/S0968-0896(02)00451-0. PMID 12538019.
- Martin BR, Jefferson R, Winckler R, Wiley JL, Huffman JW, Crocker PJ, Saha B, Razdan RK. Manipulation of the tetrahydrocannabinol side chain delineates agonists, partial agonists, and antagonists. J Pharmacol Exp Ther. 1999 Sep;290(3):1065-79. PMID 10454479
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG (June 2002). "International Union of Pharmacology. XXVII. Classification of cannabinoid receptors". Pharmacological Reviews. 54 (2): 161–202. doi:10.1124/pr.54.2.161. PMID 12037135. S2CID 8259002.
- Huffman JW, Padgett LW, Isherwood ML, Wiley JL, Martin BR (October 2006). "1-Alkyl-2-aryl-4-(1-naphthoyl)pyrroles: new high affinity ligands for the cannabinoid CB1 and CB2 receptors". Bioorganic & Medicinal Chemistry Letters. 16 (20): 5432–5. doi:10.1016/j.bmcl.2006.07.051. PMID 16889960.
- Marriott KS, Huffman JW (2008). "Recent advances in the development of selective ligands for the cannabinoid CB(2) receptor". Current Topics in Medicinal Chemistry. 8 (3): 187–204. doi:10.2174/156802608783498014. PMID 18289088.
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, Cassidy MP, Wiley JL, Martin BR (September 2005). "1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles". Bioorganic & Medicinal Chemistry Letters. 15 (18): 4110–3. doi:10.1016/j.bmcl.2005.06.008. PMID 16005223.
- Huffman JW, Bushell SM, Miller JR, Wiley JL, Martin BR (December 2002). "1-Methoxy-, 1-deoxy-11-hydroxy- and 11-hydroxy-1-methoxy-Delta(8)-tetrahydrocannabinols: new selective ligands for the CB2 receptor". Bioorganic & Medicinal Chemistry. 10 (12): 4119–29. doi:10.1016/s0968-0896(02)00331-0. PMID 12413866.
- Wiley JL, Smith VJ, Chen J, Martin BR, Huffman JW (2012). "Synthesis and pharmacology of 1-alkyl-3-(1-naphthoyl)indoles: Steric and electronic effects of 4- and 8-halogenated naphthoyl substituents". Bioorganic & Medicinal Chemistry. 20 (6): 2067–2081. doi:10.1016/j.bmc.2012.01.038. PMC 3298571. PMID 22341572.
- The Cannabinoid Receptors. The Receptors. 2009. doi:10.1007/978-1-59745-503-9. ISBN 978-1-58829-712-9.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.