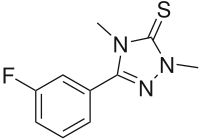
Suritozole
Suritozole (MDL 26,479) is an investigational cognition enhancer. It acts as a partial inverse agonist at the benzodiazepine receptor site on the GABAA ion channel complex, but does not have either anxiogenic or convulsant effects, unlike other BZD inverse agonists such as DMCM.[1] It was investigated for the treatment of depression and Alzheimer's disease,[2] but clinical development seems to have been discontinued.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C10H10FN3S |
| Molar mass | 223.27 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Synthesis
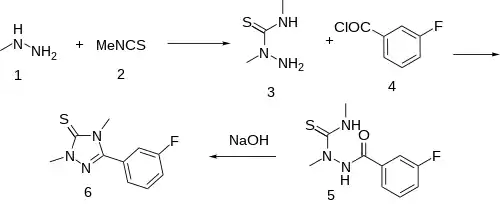
The reaction between monomethylhydrazine [60-34-4] (1) and methyl isothiocyanate (Trapex) [556-61-6] (2) gave 2,4-dimethylthiosemicarbazide [6621-75-6] (3). Amide formation with 3-fluorobenzoyl chloride [1711-07-5] (4) yielded 1-(3-fluorobenzoyl)-2,4-dimethylthiosemicarbazide [110623-52-4] (5). Cyclization to Suritozole (6).
References
- Miller JA, Dudley MW, Kehne JH, Sorensen SM, Kane JM (September 1992). "MDL 26,479: a potential cognition enhancer with benzodiazepine inverse agonist-like properties". Br. J. Pharmacol. 107 (1): 78–86. doi:10.1111/j.1476-5381.1992.tb14466.x. PMC 1907590. PMID 1330168.
- Robbins DK, Hutcheson SJ, Miller TD, Green VI, Bhargava VO, Weir SJ (May 1997). "Pharmacokinetics of MDL 26479, a novel benzodiazepine inverse agonist, in normal volunteers". Biopharm Drug Dispos. 18 (4): 325–34. doi:10.1002/(SICI)1099-081X(199705)18:4<325::AID-BDD21>3.0.CO;2-1. PMID 9158880.
- Miller, JA; Dudley, MW; Kehne, JH; Sorensen, SM; Wenstrup, DL; Kane JM;. MDL 26,479 Drugs Fut 1992, 17, 1, 21.
- Kane, John M.; Dudley, Mark W.; Sorensen, Stephen M.; Miller, Francis P. (1988). "2,4-Dihydro-3H-1,2,4-triazole-3-thiones as potential antidepressant agents". Journal of Medicinal Chemistry. 31 (6): 1253–1258. doi:10.1021/jm00401a031.
- Louks, David H.; Stolz-Dunn, Sandra K. (2007). "Kinetics for Scale-Up of a One-Pot Pathway to 5-(3-Fluorophenyl)-2,4-dihydro-2,4-dimethyl-3H-1,2,4-triazole-3-thione Using a Hybrid Model of Parallel and Consecutive Reactions". Organic Process Research & Development. 11 (5): 877–884. doi:10.1021/op700101g.
- US5856350 idem Christopher Robin Dalton, John Michael Kane, John Herr Kehne, WO 1996033177 (1999 to Hoechst Marion Roussel, Inc.).
- US5723624 idem Sandra K. Stolz-Dunn, David H. Louks, Yolanda M. Puga, Christian T. Goralski, WO 1996001812 (1998 to Merrell Pharmaceuticals Inc).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.