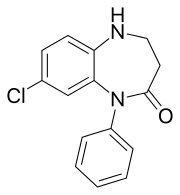
Lofendazam
Lofendazam[1] is an organic molecule which is a benzodiazepine derivative. Lofendazam is a 1,5-benzodiazepine, with the nitrogen atoms located at positions 1 and 5 of the diazepine ring; therefore, lofendazam is most closely related to other 1,5-benzodiazepines such as clobazam.[2][3]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.975 |
| Chemical and physical data | |
| Formula | C15H13ClN2O |
| Molar mass | 272.73 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Lofendazam as a human pharmaceutical has sedative and anxiolytic effects similar to those produced by other benzodiazepine derivatives. It is an active metabolite of another benzodiazepine, arfendazam.[4]
See also
References
- DE 1929656
- Malik F, Hasan M, Khan KM, Perveen S, Snatzke G, Duddeck H, Voelter W (1995). "Syntheses and CD studies of optically active substituted 1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-ones". Liebigs Annalen der Chemie. 1995 (10): 1861–1869. doi:10.1002/jlac.1995199510261.
- Aversa MC, Giannetto P, Romeo G, Ficarra P, Vigorita MG (1981). "Nuclear magnetic resonance spectra of psychotherapeutic agents. V* - conformational analysis of 1,3,4,5-tetrahydro-2H-1,5-benzodiazepin-2-ones". Organic Magnetic Resonance. 15 (4): 394–398. doi:10.1002/mrc.1270150414.
- Adrien J, Albani F, Baruzzi A, Berger M, Bixler EO, Borbeley AA, Dikeos DG, Drucker-Colin R, Montero RF, Hishikawa Y, Inoue S (11 December 1995). The Pharmacology of Sleep. Springer. ISBN 978-3-540-58961-7.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.