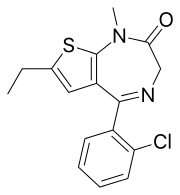
Clotiazepam
Clotiazepam[2] (marketed under brand name Clozan, Distensan, Trecalmo, Rize, Rizen and Veratran) is a thienodiazepine drug which is a benzodiazepine analog. The clotiazepam molecule differs from benzodiazepines in that the benzene ring has been replaced by a thiophene ring.[3] It possesses anxiolytic,[4] skeletal muscle relaxant,[5] anticonvulsant, sedative properties.[6] Stage 2 NREM sleep is significantly increased by clotiazepam.[7]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Veratran, Rize, Clozan |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, sublingual, liquid drops |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~90% |
| Metabolism | Hepatic |
| Elimination half-life | 4 hours[1] |
| Excretion | Renal |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.920 |
| Chemical and physical data | |
| Formula | C16H15ClN2OS |
| Molar mass | 318.82 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |

Indications
Clotiazepam has been trialed and found to be effective in the short-term management of anxiety.[8] Clotiazepam is also used as a premedicant in minor surgery in France and Japan, where the drug is commercially available under the brand names Veratran and Rize, respectively.[9][10]
Pharmacokinetics
A cross-over study in six healthy volunteers (median age 28 years) was conducted using single-dose pharmacokinetics of 5 mg clotiazepam drops, oral tablets, and sublingual tablets. The formulations had similar systemic availability. Compared with oral tablets, the sublingual route gave a lower peak concentration and a delayed peak time, while drops gave a greater maximum concentration with a similar peak time. The use of drops is suggested for a more marked initial effect and the sublingual route for easier administration, especially in the elderly.[11]
Pharmacology
Similar to other benzodiazepines clotiazepam has anxiolytic, sedative, hypnotic, amnesic, anticonvulsant and muscle relaxant pharmacological properties.[6] Clotiazepam binds to the benzodiazepine site of the GABAA receptor where it acts as a full agonist; this action results in an enhanced GABA inhibitory effect at the GABAA receptor which results in the pharmacological effects of clotiazepam.[12]
Clotiazepam has a short elimination half-life and is less prone to accumulation after repeated dosing compared to longer-acting benzodiazepine agents. It is metabolised via oxidation.[13] Clotiazepam is metabolised to hydroxy-clotiazepam and desmethyl-clotiazepam. After oral ingestion of a single 5 mg dose of clotiazepam by three healthy volunteers the drug was rapidly absorbed.[14] The elimination half-life of the drug and its metabolites range from 6.5 hours to 18 hours. Clotiazepam is 99 percent bound to plasma protein.[14] In elderly men the elimination half-life is longer and in elderly women the volume of distribution is increased.[15] Individuals with liver impairment have a reduced volume of distribution as well as a reduced total clearance of clotiazepam; renal impairment does not affect the kinetics of clotiazepam.[16]
The dose equivalent to 10 mg diazepam is thought to be between 5 and 10 mg clotiazepam.
Side effects
Side effects experienced with this product will resemble those of other benzodiazepines. Drowsiness and asthenia are common side effects.[17] There has been a report of reversible hepatitis caused by clotiazepam.[18]
Abuse
Clotiazepam is a recognised drug of abuse.[19]
See also
References
- "Clotiazépam" (PDF). HAS - Direction de l'Evaluation Médicale. Economique et de Santé Publique. 20 May 2015.
- DE 2107356, Nakanishi M, Kazuhiko A, Tetsuya T, Shiroki M, "Thieno-(2,3-E)(1,4)diazepin-2-ones", issued 3 May 1978, assigned to Yoshitomi Pharmaceutical Industries, Ltd.
- Niwa T, Shiraga T, Ishii I, Kagayama A, Takagi A (September 2005). "Contribution of human hepatic cytochrome p450 isoforms to the metabolism of psychotropic drugs". Biological & Pharmaceutical Bulletin. 28 (9): 1711–1716. doi:10.1248/bpb.28.1711. PMID 16141545.
- Klicpera C, Strian F (May 1978). "Autonomic perception and responses in anxiety-inducing situations". Pharmakopsychiatrie, Neuro-Psychopharmakologie. 11 (3): 113–120. doi:10.1055/s-0028-1094569. PMID 27828.
- Fukuda T, Tsumagari T (August 1983). "Effects of psychotropic drugs on the rage responses induced by electrical stimulation of the medial hypothalamus in cats". Japanese Journal of Pharmacology. 33 (4): 885–890. doi:10.1254/jjp.33.885. PMID 6632385.
- Mandrioli R, Mercolini L, Raggi MA (October 2008). "Benzodiazepine metabolism: an analytical perspective". Current Drug Metabolism. 9 (8): 827–844. doi:10.2174/138920008786049258. PMID 18855614.
- Nakazawa Y, Kotorii M, Oshima M, Horikawa S, Tachibana H (October 1975). "Effects of thienodiazepine derivatives on human sleep as compared to those of benzodiazepine derivatives". Psychopharmacologia. 44 (2): 165–171. doi:10.1007/BF00421005. PMID 709. S2CID 13365554.
- Martucci N, Manna V, Agnoli A (April 1987). "A clinical and neurophysiological evaluation of clotiazepam, a new thienodiazepine derivative". International Clinical Psychopharmacology. 2 (2): 121–128. doi:10.1097/00004850-198704000-00005. PMID 2885366.
- "RIZE TABLETS 5mg". Official Japanese Drug Information Sheet (Kusuri-no-Shiori). February 2016.
- "Clotiazepam (Veratran)". French Guide to Medicines.
- Benvenuti C, Bottà V, Broggini M, Gambaro V, Lodi F, Valenti M (1989). "The pharmacokinetics of clotiazepam after oral and sublingual administration to volunteers". European Journal of Clinical Pharmacology. 37 (6): 617–619. doi:10.1007/BF00562556. PMID 2575522. S2CID 29397932.
- Yakushiji T, Fukuda T, Oyama Y, Akaike N (November 1989). "Effects of benzodiazepines and non-benzodiazepine compounds on the GABA-induced response in frog isolated sensory neurones". British Journal of Pharmacology. 98 (3): 735–740. doi:10.1111/j.1476-5381.1989.tb14600.x. PMC 1854765. PMID 2574062.
- Greenblatt DJ, Divoll M, Abernethy DR, Ochs HR, Shader RI (1983). "Clinical pharmacokinetics of the newer benzodiazepines". Clinical Pharmacokinetics. 8 (3): 233–252. doi:10.2165/00003088-198308030-00003. PMID 6133664. S2CID 19691487.
- Arendt R, Ochs HR, Greenblatt DJ (1982). "Electron capture GLC analysis of the thienodiazepine clotiazepam. Preliminary pharmacokinetic studies". Arzneimittel-Forschung. 32 (4): 453–455. PMID 6125154.
- Ochs HR, Greenblatt DJ, Verburg-Ochs B, Harmatz JS, Grehl H (1984). "Disposition of clotiazepam: influence of age, sex, oral contraceptives, cimetidine, isoniazid and ethanol". European Journal of Clinical Pharmacology. 26 (1): 55–59. doi:10.1007/BF00546709. PMID 6143670. S2CID 44321356.
- Ochs HR, Greenblatt DJ, Knüchel M (1986). "Effect of cirrhosis and renal failure on the kinetics of clotiazepam". European Journal of Clinical Pharmacology. 30 (1): 89–92. doi:10.1007/BF00614202. PMID 2872061. S2CID 21304989.
- Colonna L, Cozzi F, Del Citerna F, Di Benedetto A, De Divitiis O, Furlanello F, et al. (1990). "[Multicenter study of the effectiveness and tolerance of clotiazepam in cardiology]". Minerva Cardioangiologica. 38 (1–2): 45–49. PMID 1971433.
- Habersetzer F, Larrey D, Babany G, Degott C, Corbic M, Pessayre D, Benhamou JP (September 1989). "Clotiazepam-induced acute hepatitis". Journal of Hepatology. 9 (2): 256–259. doi:10.1016/0168-8278(89)90060-3. PMID 2572625.
- Shimamine M, Masunari T, Nakahara Y (1993). "[Studies on identification of drugs of abuse by diode array detection. I. Screening-test and identification of benzodiazepines by HPLC-DAD with ICOS software system]". Eisei Shikenjo Hokoku. Bulletin of National Institute of Hygienic Sciences (111): 47–56. PMID 7920567.