Menthyl isovalerate
Menthyl isovalerate, also known as validolum, is the menthyl ester of isovaleric acid. It is a transparent oily, colorless liquid with a smell of menthol. It is very slightly soluble in ethanol, while practically insoluble in water. It is used as a food additive for flavor and fragrance.[1] It is produced by typical esterification procedures with usual catalysts such as HCl or H2SO4, and is rather slow, usually done for 20+hr at 100°C or a bit higher. Validol, the anxiety medication containing a roughly 25% solution of menthol in menthyl isovalerate is prepared essentially in one step, in which the amount of menthol added before conducting the acid catalysed esterification is in an excess such that the resulting solution of the yielded ester has around 25% menthol, simplifying the procedure. Work up might consist of several washings, including one with aqueous sodium bicarbonate to neutralize traces of acid catalyst and unreacted isovaleric acid, and distillation.
 | |
| Names | |
|---|---|
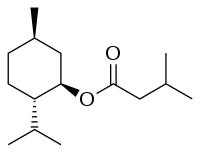
| Preferred IUPAC name
(1R,2S,5R)-5-Methyl-2-(propan-2-yl)cyclohexyl 3-methylbutanoate | |
| Other names
Validolum; Valofin; Validol; Menthoval | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.036.766 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C15H28O2 |
| Molar mass | 240.387 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Medical use
In Poland, Bulgaria, and the former Soviet Union states including Russia, menthyl isovalerate mixed with roughly 25% menthol is sold as an anxiolytic under various trade names including Validol, Valofin, and Menthoval.[2][3][4]
See also
- Methyl pentanoate
- Hopantenic acid
- Isovaleramide
References
- Menthyl isovalerate
- Russian Medications List and Possible Side Effects
- "Farmak Product Information - Validol" (PDF). Archived from the original (PDF) on 2016-03-15. Retrieved 2013-04-05.
- Bondarenko IP, Liashenko MM, Chirkov SN, Ermakovich II (1996). "A comparative evaluation of the antianginal action of commercially and noncommercially produced validol in neurocirculatory dystonia and stenocardia". Lik Sprava. Mar–Apr (3–4): 110–113. PMID 9035841.