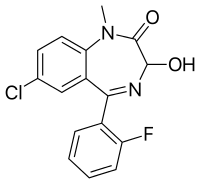
Flutemazepam
Flutemazepam, initially first synthesized in 1965,[1] but wasn't further developed and described until a team at Stabilimenti Chimici Farmaceutici Riuniti SpA in the mid 1970s.[2][3] It is the fluorinated analogue of temazepam that has powerful hypnotic, sedative, amnesiac, anxiolytic, anticonvulsant and skeletal muscle relaxant properties.[4] It is most closely related in structure to temazepam, being the fluoro instead of chloro analogue. As a result, flutemazepam has been shown to have similar pharmacological properties to temazepam and nimetazepam. It has been found to be effective for the treatment of the most severe states of anxiety, panic attacks, and severe insomnia. Furthermore, it is highly potent with 1 mg of flutemazepam being equivalent to 10 mg of diazepam.[5][6][7][8] Flutemazepam is highly effective for acute psychotic states, especially stimulant psychosis, violent behaviour, and aggression.[9]
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.052.612 |
| Chemical and physical data | |
| Formula | C16H12ClFN2O2 |
| Molar mass | 318.730 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It was first synthesized and described in 1965 by Leo Sternbach.[1] In a test which compared a series of 3-fluorobenzodiazepine compounds in 1976, one of which was the 3-hydroxy benzodiazepine, flutemazepam. Of the tested compounds (9 different 3-fluorobenzodiazepines including, a powerful compound known as N-Desalkyl-3-hydroxyflurazepam). Flutemazepam was the most potent: 20x more potent than temazepam, 10x more potent than diazepam and nitrazepam, 5x more potent than nimetazepam and roughly equipotent to a related 3-hydroxy benzodiazepine, lorazepam. It was also the most rapid-acting compound of the series via oral administration, as well as the most active and powerful anxiolytic, myorelaxant, tranquilizer, motor-impairing, amnesic, sedative-hypnotic, and anti-convulsive agent at doses as low as 0.5-1 mg range.[10]
See also
- Benzodiazepine
- Nitemazepam - 7-nitro analogue of temazepam
- Temazepam
References
- "PubChem Compound Summary for CID 40344, Flutemazepam". National Center for Biotechnology Information (2022). Retrieved 30 July 2022.
- GB 1431282, "Benzodiazepine Compounds for Therapeutical Use", issued 7 April 1976, assigned to Schiapparelli Farma SpA
- "Flutemazepam". PubChem. National Library of Medicine. Retrieved 2022-03-31.
- "Universal Guide for Temazepam". fastukmeds.com. Retrieved 2022-03-31.
- "Flutemazepam". US Biologic. United States Biological. Retrieved 22 July 2022.
- "Temazepam Oral: Uses, Side Effects, Interactions, Pictures, Warnings & Dosing - WebMD". www.webmd.com. Retrieved 2022-03-31.
- "flutemazepam". psychotropics.dk. Retrieved 11 February 2009.
- Morton IK, Hall JM (1999). Concise Dictionary of Pharmacological Agents. Springer. ISBN 978-0-7514-0499-9.
- Esmailian M, Ahmadi O, Taheri M, Zamani M (September 2015). "Comparison of haloperidol and midazolam in restless management of patients referred to the Emergency Department: A double-blinded, randomized clinical trial". Journal of Research in Medical Sciences. 20 (9): 844–849. doi:10.1002/14651858.CD003079.pub4/14651858.CD003079.pub4. PMC 6486117.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Bingham, EM. "Process for the preparation of 3-fluorobenzodiazepines". Google Patents. US Patent US4120856A. Retrieved 30 July 2022.