Suriclone
Suriclone (Suril) is a sedative and anxiolytic drug in the cyclopyrrolone family of drugs. Other cyclopyrrolone drugs include zopiclone and pagoclone.
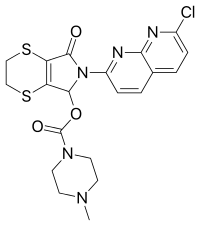 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.053.431 |
| Chemical and physical data | |
| Formula | C20H20ClN5O3S2 |
| Molar mass | 477.98 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Suriclone has a very similar pharmacological profile to the benzodiazepine family of drugs including sedative and anxiolytic properties but with less amnestic effects,[1][2] and its chemical structure is quite different from that of the benzodiazepine drugs.
The mechanism of action by which suriclone produces its sedative and anxiolytic effects is by modulating GABAA receptors, although suriclone is more subtype-selective than most benzodiazepines.[3]
References
- Gilburt SJ, Fairweather DB, Kerr JS, Hindmarch I (1992). "The effects of acute and repeated doses of suriclone on subjective sleep, psychomotor performance and cognitive function in young and elderly volunteers". Fundamental & Clinical Pharmacology. 6 (6): 251–8. doi:10.1111/j.1472-8206.1992.tb00118.x. PMID 1362556. S2CID 23320665.
- Saletu B, Grünberger J, Linzmayer L, Semlitsch HV, Anderer P, Chwatal K (February 1994). "Pharmacokinetic and -dynamic studies with a new anxiolytic, suriclone, utilizing EEG mapping and psychometry". British Journal of Clinical Pharmacology. 37 (2): 145–56. doi:10.1111/j.1365-2125.1994.tb04254.x. PMC 1364591. PMID 7910470.
- Blanchard JC, Julou L (March 1983). "Suriclone: a new cyclopyrrolone derivative recognizing receptors labeled by benzodiazepines in rat hippocampus and cerebellum". Journal of Neurochemistry. 40 (3): 601–7. doi:10.1111/j.1471-4159.1983.tb08023.x. PMID 6298365. S2CID 35732735.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.