Apimostinel
Apimostinel (GATE-202, formerly NRX-1074) is an investigational antidepressant, acting as a novel and selective modulator of the NMDA receptor.[1][2][3][4] It is currently under development for the acute treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Naurex and Allergan.[5][6][7] As of February 2015, an intravenous formulation of apimostinel has completed a phase IIa clinical trial for MDD.[5][8]
 | |
| Clinical data | |
|---|---|
| Other names | NRX-1074; AGN-241660; Threonyl-prolyl-2R-(2-benzyl)-prolyl-threonine amide |
| Routes of administration | By mouth |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
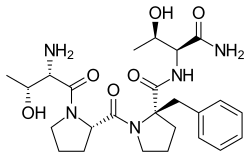
| Formula | C25H37N5O6 |
| Molar mass | 503.600 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Similar to rapastinel (GLYX-13), its mechanism of action acts through a unique binding site on the NMDA receptor, independent of the glycine site, to modulate receptor activity and enhance NMDAR-mediated synaptic plasticity.[9] However, apimostinel is 1000-fold more potent in vitro and is intended as an improved, follow-up drug to rapastinel.[2][5] Similar to rapastinel, apimostinel is an amidated tetrapeptide, but has been structurally modified, via the addition of a benzyl group, to enhance its metabolic stability and pharmacokinetic profile. The drug has shown rapid and potent antidepressant effects in pre-clinical models of depression.[5] In addition, similarly to rapastinel, it is well tolerated and lacks the schizophrenia-like psychotomimetic effects of NMDA receptor antagonists such as ketamine.[5]
References
- PR Newswire (2010). "Naurex's Novel Antidepressant GLYX-13 Recognized as One of Windhover's Top 10 Neuroscience Projects to Watch".
- Henter ID, Park LT, Zarate CA (May 2021). "Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status". CNS Drugs. 35 (5): 527–543. doi:10.1007/s40263-021-00816-x. PMC 8201267. PMID 33904154.
- Donello JE, Banerjee P, Li YX, Guo YX, Yoshitake T, Zhang XL, et al. (March 2019). "Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects". The International Journal of Neuropsychopharmacology. 22 (3): 247–259. doi:10.1093/ijnp/pyy101. PMC 6403082. PMID 30544218.
- Hayley S, Litteljohn D (November 2013). "Neuroplasticity and the next wave of antidepressant strategies". Frontiers in Cellular Neuroscience. 7: 218. doi:10.3389/fncel.2013.00218. PMC 3834236. PMID 24312008.
- PR Newswire (2014). "Naurex Reports Positive Top-Line Phase 2b Results for Novel Antidepressant GLYX-13 and Advances NRX-1074 into Phase 2 Depression Study".
- plc, Allergan. "Allergan Successfully Completes Naurex Acquisition". www.prnewswire.com. Retrieved 2016-11-20.
- "Home - Gate Neurosciences". Retrieved 2022-05-12.
- "Study of Intravenous NRX-1074 in Patients With Major Depressive Disorder". Clinicaltrials.gov. US National Institutes of Health. Retrieved 10 December 2014.
- Donello, John E.; Banerjee, Pradeep; Li, Yong-Xin; Guo, Yuan-Xing; Yoshitake, Takashi; Zhang, Xiao-Lei; Miry, Omid; Kehr, Jan; Stanton, Patric K.; Gross, Amanda L.; Burgdorf, Jeffery S. (2019-03-01). "Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects". The International Journal of Neuropsychopharmacology. 22 (3): 247–259. doi:10.1093/ijnp/pyy101. ISSN 1469-5111. PMC 6403082. PMID 30544218.