Lanicemine
Lanicemine (AZD6765) is a low-trapping NMDA receptor antagonist that was under development by AstraZeneca for the management of severe and treatment-resistant depression.[1][2] Lanicemine differs from ketamine in that it is a low-trapping NMDA receptor antagonist, showing similar rapid-acting antidepressant effects to ketamine in clinical trials but with little or no psychotomimetic side effects.[3] However, lanicemine did not meet study endpoints, and its development was terminated by AstraZeneca in 2013.[4]
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
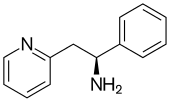
| Formula | C13H14N2 |
| Molar mass | 198.269 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
See also
References
- "Lanicemine". AdisInsight. Retrieved 18 June 2017.
- Machado-Vieira R, Henter ID, Zarate CA (May 2017). "New targets for rapid antidepressant action". Progress in Neurobiology. 152: 21–37. doi:10.1016/j.pneurobio.2015.12.001. PMC 4919246. PMID 26724279.
- Zarate CA, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, et al. (August 2013). "A randomized trial of a low-trapping nonselective N-methyl-D-aspartate channel blocker in major depression". Biological Psychiatry. 74 (4): 257–64. doi:10.1016/j.biopsych.2012.10.019. PMC 3594049. PMID 23206319.
- Flowers S. "Return to growth: AstraZeneca's CEO Pascal Soriot says 2013 was year of "momentum" for the company". Retrieved 6 February 2014.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.