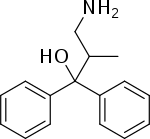
2-MDP
2-MDP (U-23807A) is a dissociative anaesthetic drug which has been found to be an NMDA antagonist and produces similar effects to PCP in animals. The levo or (-) isomer is the active form of the drug.[1][2] It also has stimulant effects, having only around one third the potency of amphetamine by weight, but with a long duration of action, lasting more than 24 hours from a single oral dose.[3]
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H19NO |
| Molar mass | 241.334 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Effects
The therapeutic action is said to exhibit appetite suppressant (c.f. patent), and antidepressant[4] like activity.
Synthesis
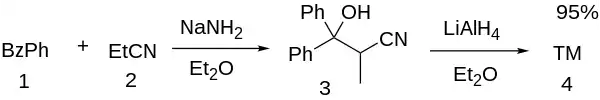
In a variation of the nitrile-Aldol reaction (also demonstrated for venlafaxine & clofedanol), combination of benzophenone (1) and propionitrile (2), in the presence of sodamide base and ethyl ether solvent, leads to 3-hydroxy-2-methyl-3,3-diphenylpropanenitrile [6275-86-1] (3). The reduction of the intermediate nitrile group with lithium aluminium hydride completed the synthesis of U-23,807A (4).
See also
Various analogs are available:
- BRN 2814419 [4150-90-7]
- BRN 1322519 [4082-37-5]
- BRN 2819660 [2104-80-5]
- SK&F 70463-A HCl: [1477-79-8]
References
- Tang AH, Cangelosi AA, Code RA, Franklin SR (February 1984). "Phencyclidine-like behavioral effects of 2-methyl-3,3-diphenyl-3-propanolamine (2-MDP)". Pharmacology, Biochemistry, and Behavior. 20 (2): 209–13. doi:10.1016/0091-3057(84)90244-2. PMID 6718449. S2CID 38908019.
- Blake JC, Davies SN, Church J, Martin D, Lodge D (January 1986). "2-Methyl-3,3-diphenyl-3-propanolamine (2-MDP) selectively antagonises N-methyl-aspartate (NMA)". Pharmacology, Biochemistry, and Behavior. 24 (1): 23–5. doi:10.1016/0091-3057(86)90038-9. PMID 3511477. S2CID 29762524.
- Biel JH (January 1967). Cain CK (ed.). "Antidepressants, Stimulants, Hallucinogens". Annual Reports in Medicinal Chemistry. Academic Press. 2: 11–23, 18. doi:10.1016/S0065-7743(08)61499-2.
- Shipley GS, Bishop MP, Gallant DM. A controlled evaluation of U-23,807A in the neurotic depressive syndrome. Curr Ther Res Clin Exp. 1967 Oct;9(10):514-6. PMID: 4964946.
- Moffett, R. B., Pickering, T. L. (November 1971). "Central nervous system agents. 2. Synthesis of diphenyl primary and secondary aminopropanols". Journal of Medicinal Chemistry. 14 (11): 1100–1106. doi:10.1021/jm00293a019. ISSN 1520-4804 0022-2623, 1520-4804.
{{cite journal}}: Check|issn=value (help)
| Adamantanes | |
|---|---|
| Adenosine antagonists |
|
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cathinones |
|
| Cholinergics |
|
| Convulsants | |
| Eugeroics |
|
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines |
|
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines |
|
| Others |
|
| AMPAR |
|
|---|---|
| KAR |
|
| NMDAR |
|
| |